Social media is transforming our understanding of drug safety
Posted: 17 November 2021 | Daniel Ghinn (CREATION.co) | No comments yet
Here, Daniel Ghinn of CREATION.co discusses the growing role social media analysis is playing in helping to provide pharmaceutical companies and health regulators with real-world evidence on drug safety.


Social media has transformed people’s lives. From Twitter to Tumblr, it is estimated that by 2025 more than half the world’s population (4.41 billion people) will use social media in some form. As engagement rises so too does the level of information shared. Advancements in our capacity to monitor and interpret this information is also improving.
The ability to interpret this data holds value across industries. Healthcare is no exception. Pharmaceutical companies and regulators can now harness the sentiments of ‘empowered patients’ and healthcare professionals online to better understand conversations relevant to specific drugs and treatments. This knowledge is already being used to strengthen drug safety information by helping to uncover real-world cases, improving our understanding of how drugs are being used, and shedding light on little-known side effects and drug interactions.
To explore social media’s potential to provide information on drug safety, below I explore an example.
Gepants
Online analysis of sentiment expressed by patients and healthcare professionals on social media towards gepants, a new form of migraine treatment, showcases the powerful applications of this research.


Migraines are estimated to affect around one in seven people, making them the third most common disease in the world. Triptans, a group of 5-HT receptor agonists, have been widely and successfully used for the acute treatment of migraines since the 1990s. In December 2019, gepants, a new class of migraine treatment entered the market with Allergan’s Ubrelvy, the first CGRP receptor antagonist to gain US Food and Drug Administration (FDA) approval. The new class has been shown to be safe and effective with the added benefit of use by people with vascular disease, heart disease and stroke. Clinical trials are now positioning gepants as a suitable alternative to triptans, the established drug class.
Side Effects
To gain insight into both patients’ and clinicians’ views of the relative safety of Ubrelvy as a new offering, we analysed all healthcare professional, patient and public conversations worldwide relating to migraine drugs on all open social media platforms between 1 January 2020 and 31 March 2021. Of these conversations, we identified and collected conversations mentioning Ubrelvy, a triptan, or an unspecified migraine drug. From this dataset of 98,110 mentions, we further explored conversations relating to common side-effects as well as less well-known side effects identified through exploratory conversation analysis.
Across the seven triptans, Ubrelvy and conversations that referred to a migraine drug without specifying a particular product, side effects made up one-fifth (22 percent) of the conversations. Axert received the lowest total number of posts (492), but the highest percentage of side effect mentions (40 percent).
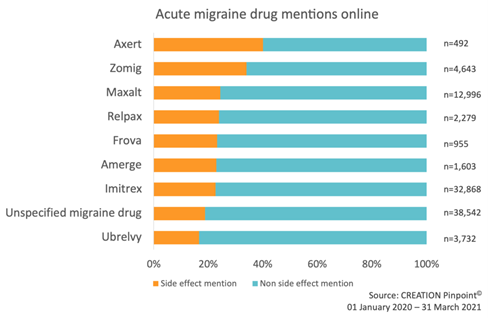

Figure 1: Percentage of migraine drug conversations that mention a side effect and do not mention a side effect. Data includes conversations from HCPs, patients and the public on open social media sites.
Establishing the level of conversation around side effects is an essential tool in understanding product perception. From this framework we set out to accurately isolate the voice of individuals who have taken a product from those simply talking about the drug. Typically, these are individuals whose experiences offer greatest insight into the safety profile of a drug and that can provide early drug safety signals to pharmaceutical manufacturers.
Identifying patient cases
Further analysis enabled the separation of individual patient experiences from the noise of wider side effect conversation. These patient experiences were primarily reported on Twitter and Reddit, with several other blogging and health platforms being used.
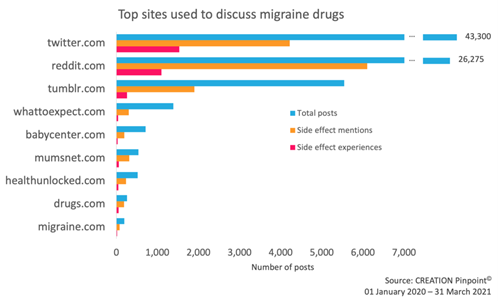

Figure 2: Social media platforms used to discuss migraine drugs. Patients shared their experiences with migraine drugs mostly on Twitter and Reddit. Twitter was the most common platform for mentioning migraine drugs, but side effects were more often mentioned on Reddit.
Lived patient experiences made up around 17 percent of the total mentions about migraine drug side effects from healthcare professionals, patients and the public. Excluding side effect conversations that did not contain patient experiences, we observed that nausea was most commonly reported by patients. The finding that a commonly experienced side effect was commonly reported online may in and of itself be unremarkable. However, further analysis revealed other events of varying degrees of severity. Patients’ frequently sought advice and reassurance from others who may be experiencing the same issues.
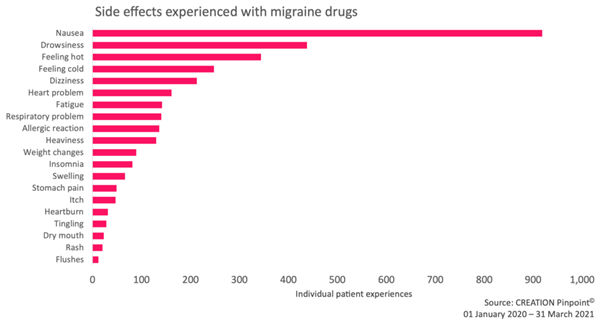

Figure 3: Drug side effects experienced by migraine patients, as expressed on social media. Migraine drugs refer to Ubrelvy, a triptan or an unspecified migraine drug.
Uncommon Side Effects
Beyond nausea and other common side effects, we also identified patient conversations that expressed side effects rarely mentioned on patient information websites. For instance, we identified 81 patient cases of insomnia – more than the number of patients reporting to have experienced commonly listed side effects such as itches, tingling, rashes and swelling.
Drowsiness is commonly listed on patient information sites and was the second most reported side effect online with 438 patient cases. Insomnia, as a rarely acknowledged side effect, represents an underrepresented group with respect to medical information online. Some patients expressed difficulties finding relevant information. This lack of guidance online prompted them to seek reassurance that they are not alone.
That insomnia was mentioned in association with the first-in-class gepant may point toward an issue that goes beyond the lack of information about migraine treatments and insomnia and perhaps suggests that this patient experience is not well recognised by the medical community.
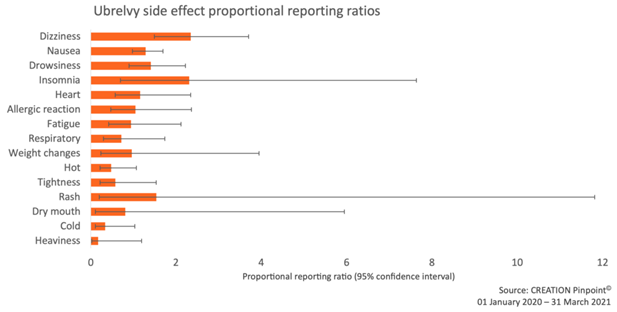

Figure 4: Proportional reporting ratios (PRR) of Ubrelvy side effects using the Triptans class as comparator. Insomnia PRR = 2.3 (0.7 – 7.6).
A PRR of 2.3 indicates that for the insomnia-Ubrelvy pair, the probability of reporting insomnia with Ubrelvy rather than another side effect is 2.3 times higher compared to the probability for triptans. The matter is complicated by the wide confidence intervals (in 95 percent of cases the true PRR will fall between 0.7 and 7.6) bringing a degree of uncertainty into any conclusion. However, that experiences of insomnia are being reported online more than expected suggests that further investigation would be worthwhile.
Beyond side effects: product complaints, drug interactions and other events
Our exploration into migraine drug side-effect patient cases is just the tip of the iceberg. The opportunity to understand the profile, experience and safety of a drug goes beyond the elements of pharmacovigilance investigated here. Our research also uncovered relevant conversations around drug safety including package complaints, potential drug interactions, off-label and drug use during pregnancy.
Social media and the collation of real-world evidence for drug safety monitoring
Social media has been shown to be a valuable tool for understanding the needs and behaviours of groups across a broad range of industries, including healthcare and pharmaceuticals. There is huge potential for collecting real-world evidence for drug safety monitoring and beyond. From lesser-known side effects and drug use in particular patient groups to off label use and drug interaction, social media is providing the resources required to gain drug safety profile insight, inform product decisions, and improve patient safety and experiences.
About the author
Daniel Ghinn is the Founder and CEO of CREATION.co, which provides insights and consulting to inform health strategy, communications and policymaking among some of the world’s largest healthcare companies, government organisations and NGOs.
Related topics
Drug Markets, Drug Safety, Industry Insight, Informatics, Therapeutics









