Formulation development of vitamin C-based effervescent blend suitable for direct compression
Posted: 29 October 2021 | Caterina Funaro (IMA Active), Fabriano Ferrini (IMA Active), Federica Giatti (IMA Active) | No comments yet
This article presents the findings from a study to establish optimum formulation parameters for effective effervescent vitamin C tablet production.


Introduction
Oral solid dosage forms are the most used means of medication administration due to their ease of assumption, transport and greater stability. Conventionally, tablets are more common because, with good formulation, the manufacturing process is simple and relatively low cost.1
One of the most challenging product development stages for formulators is designing a multivitamin formulation that is as stable as possible”
Active pharmaceutical ingredients (APIs) or supplements delivered through oral dosage forms may encounter the risk of low absorption, but this may be overcome by administering the drug in liquid form. However, because many APIs show limited stability in liquid form, effervescence application has provided a good solution; drug dissolution and immediate consumption enables faster and more complete absorption compared to conventional tablets. Several advantages should be considered, including improved patient compliance (since there is no need to swallow tablets), less stomach and intestinal irritation, accurate dosing and more reproducible pharmacokinetics, to name a few. However, process manufacturing and blend formulation require some specific arrangements in the effervescent field:
- Larger tablets
- Complex production and need for specialist packaging materials
- Tasteless and water-soluble lubricants that are non-toxic.
Lubricant is, for this reason, a critical element that requires thorough evaluation. Magnesium stearate, as with other water-insoluble lubricants, prevents obtaining a clear transparent solution and retards the disintegration and dissolution that is required to be rapid in effervescent tablets.2
One of the most challenging product development stages for formulators is designing a multivitamin formulation that is as stable as possible, as several adverse effects can occur due to vitamins’ instability, which is more difficult to counter in aqueous forms than in solid or oily products. Many formulations showed that there is no significant influence of the auxiliaries (binders, fillers, disintegrants, etc) on the stability of the vitamins themselves, provided the free water content is limited. The best way to process vitamins, therefore, is direct compression to eliminate water addition, as in granulation. As such, the ideal formulation must be thoroughly investigated; the combination of excipient and API or supplements in general should have guaranteed acceptable flowability and compressibility while simultaneously ensuring tablet disaggregation.
The aim of our study was to define a formulation for effervescent vitamin C tablets (ascorbic acid) that is suitable for direct compression. A particular focus of the analysis was the filler selection, as it is well known4 that sugar, fructose, sorbitol or glucose delay vitamin C hydrolysis and each can cause instability. Once the type of filler and its percentage had been chosen, the aim of our experimentation was to maximise tablet press output while maintaining tablet quality.
Materials and methods
Effervescent ascorbic acid formulations (Table 1) have been well explored thanks to the close co-operation of IMA and Polaris. Their aim was to obtain an equilibrium between flowability and compressibility. 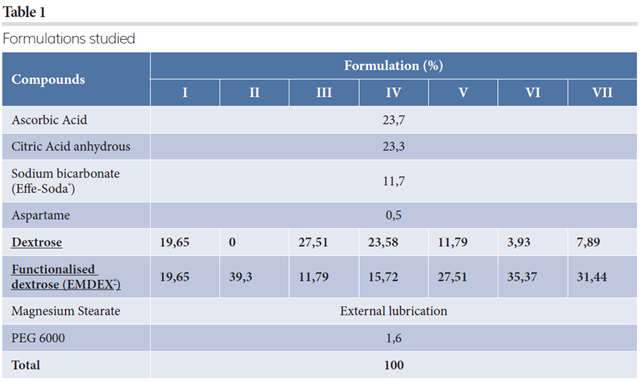



Figure 1: PREXIMA 300, IMA
First, with each formulation studied, tableting was performed at 50 rpm as turret speed with comparable values of pre-compression force (7kN) and main compression force (30kN). The formulations were identified as optimal due to the tablet strength obtained and effervescence behaviour (disintegration time in particular). The selected formulations have been optimally tableted in terms of output, ensuring consistency of the most critical process parameters. The characteristics of the final tablets and process stability were assessed to ascertain whether the aims of the study had been achieved. Weight and tablet consistency, as well as compression relative standard deviation were the most important parameters addressed. In addition, disaggregation time was always monitored to double-match the formulation choice.
Results
Tablets were always obtained with low standard deviation on weight and with standard tablet press configuration; this means that globally the formulations are suitable for direct compression. During preliminary tests, the use of Effer-Soda (SPI Pharma, Germany) was useful in developing a formulation for direct compression owing to the surface modification at sodium bicarbonate particles to enhance stability, which increased resistance to humidity and prevented premature effervescence.5 Adding magnesium stearate (Brenntag, Italy) through the external approach, rather than blended inside the formulation, increases the average tablet strength and prevents the capping defect: LUMS (IMA, Italy) allows formulators to use the minimum possible to prevent sticking onto dies and punches, as previously studied.6 Technological features (Carr Index and LOD) measured for Formulation II were six percent and 2.45 percent, respectively, compared to seven percent and 2.84 percent for Formulation VI. Particle size distribution (Figure 2) reflects the different percentage of excipients used.
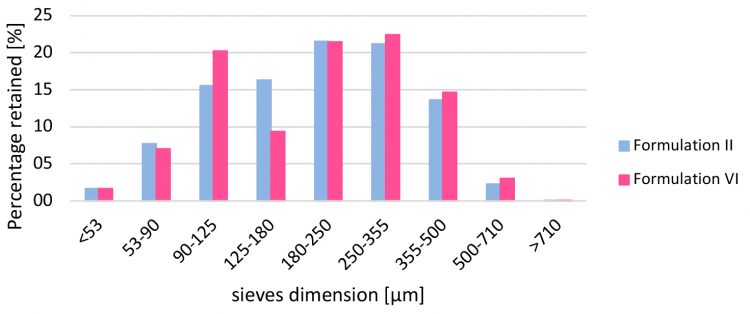

Figure 2: Particle size distribution comparison for Formulation II and VI.
To confirm the choice in terms of filler concentration, tablet strength was analysed compared to EMDEX percentage for each formulation mentioned (Figure 3).
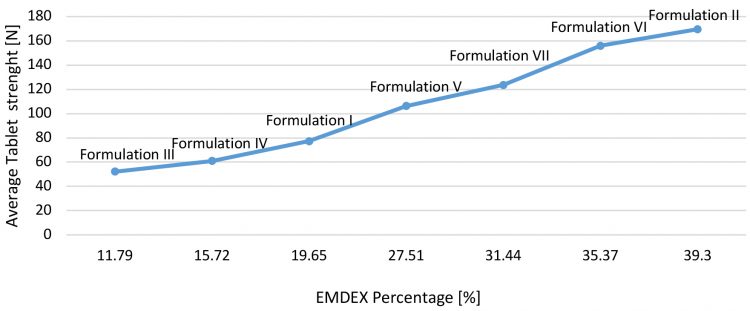

Figure 3: EMDEX percentage versus tablet strength at same turret press speed.
Same turret speed (50 rpm) was first analysed at the same pre-compression and main compression force (respectively, 7kN and 30kN); the only variable was the concentration of functionalised glucose (EMDEX) inside the formulation itself. It is clear that by increasing the EMDEX percentage, the crushing strength of tablets is enhanced, achieving good and defect-free tablets. For low concentration, tablets are pressed with capping tendency and unacceptable hardness, as represented in Figure 3. To achieve high machine output, it is advisable to use at least 90 percent EMDEX. For this reason, only Formulation II and VI were used as references to study tabletability and effervescence behaviour. More details of these two formulations were the focus of the second part of this case study (Table 2). 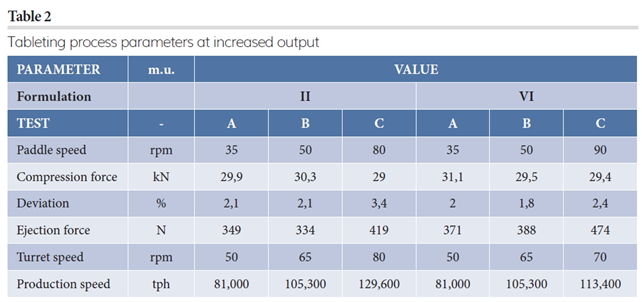

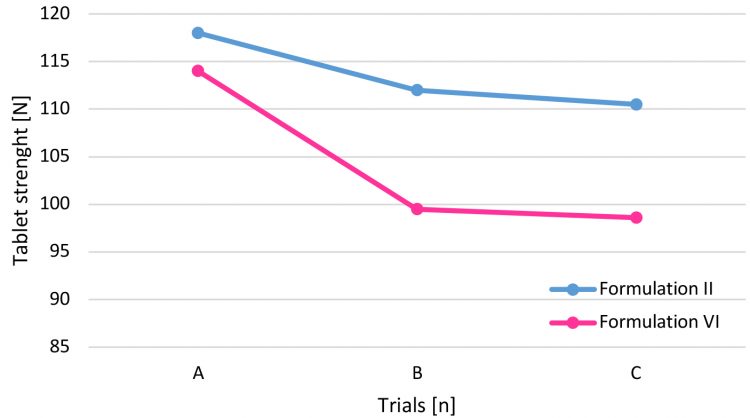

Figure 4: Tablet strength trend.
Disintegration time has also been monitored for the three tests performed (A, B and C) (Figure 5).
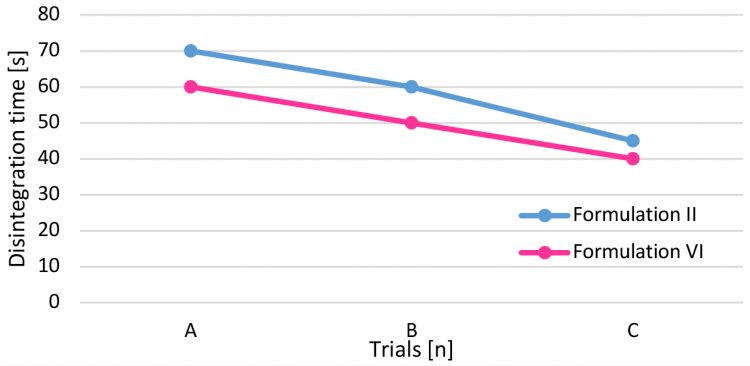

Figure 5: Disintegration trend.
Trial A and B were performed at the same turret press speed: the presence of dextrose, also in small quantities, lowers the disintegration time. The main reason could be attributed to the slightly different crushing strength obtained for the two formulations: the higher the tablet strength, the higher the disintegration time. Even if respected, the trend also applied in Test C, ie, the difference in the time could be due to the turret speed applied: 80 rpm for Formulation II and 70 rpm for Formulation VI. Increased dwell time, clearly obtained at lower turret press speed, reduced the porosity of intra-particles and slowed down the disintegration time as well.
Conclusions
Our study demonstrated that an optimal effervescent vitamin C formulation suitable for direct compression could be developed. The use of the external lubrication approach facilitated an increase in the tablet strength, minimising defects such as capping. The choice of filler resulted to be crucial: functionalised excipients such as EMDEX improved tablet quality while maintaining an acceptable effervescent behaviour. Furthermore, the use of a functionalised sugar enabled an increase in tablet press output while ensuring process stability and reliability.
About the authors






References
- Srinath, et al. IJPRD, 2011; Vol 3(3): 12; May 2011 (76-104)
- Giatti F. https://ima.it/pharma/leading-the-way/
- Buhler V. Vademecum for Vitamins Formulations, 2nd edition (75-78)
- Buhler V. Vademecum for Vitamins Formulations, 2nd edition (78-79)
- https://www.spipharma.com/en/products/functional-excipients/ effer-soda/
- Giatti F, Consoli SF, Bang F, et al. Evaluating the performance of external lubrication to convert the pre-formulated Ibuprofen DC 85 W into a ready-to-use tableting product, 3rd European Conference on Pharmaceutics, March 25-26, 2019, Bologna, Italy
Issue
Related topics
Active Pharmaceutical Ingredient (API), Drug Development, Drug Manufacturing, Formulation, Ingredients, Manufacturing, Therapeutics









