Improving infusion dosing accuracy for patient safety
Posted: 26 August 2021 | Elsa Batista (Portuguese Institute for Quality [IPQ]), Emmelyn Graham (TÜV SÜD National Engineering Laboratory) | No comments yet
Infusion is the most used technology in hospitals. Given its widespread application, often in critical situations and by many users, infusion errors are frequently made, with some having severe effects. Here, Emmelyn Graham, Flow Measurement Engineer at TÜV SÜD National Engineering Laboratory, and Elsa Batista, Head of Volume and Flow Laboratory at the Portuguese Institute for Quality (IPQ), explain the pressing need to prevent these adverse incidents by facilitating better understanding of the equipment for users.

THERE HAVE BEEN numerous injuries, deaths and adverse health effects associated with the use of infusion pumps, which have highlighted significant safety issues.1 For example, approximately 80-90 percent of hospitalised patients in the UK receive intravenous (IV) therapy using infusion devices to deliver medication, fluids and nutrients.2,3 It is extremely important that the delivery of medication and other fluids is precisely controlled over time and that the delivered dose is accurately known, especially for critical drugs at high concentration.
There have been numerous injuries, deaths and adverse health effects associated with the use of infusion pumps, which have highlighted significant safety issues.”
A well-defined metrological infrastructure is needed to ensure that infusion pump manufacturers include robust information on the ‘real’ dose delivered to the patient and to ensure users of drug delivery devices have an enhanced metrological knowledge of these critical devices, thus preventing incorrect measurements and significantly improving patient safety.
Metrology (the study of measurement) can bridge the knowledge gap by designing a representative multi-infusion intravenous system for investigating how different liquids mix and how this affects drug concentrations. The increasing uptake of novel microfluidic applications in healthcare requires the development of a metrological infrastructure for validating quality and reproducibility.
A joint research project funded under the EMPIR programme of the European Commission (EC), Metrology for Drug Delivery (MeDD II),4 began in June 2019 and involves 15 partners working collaboratively for a three-year period. Co‑ordinated by the Portuguese Institute of Quality (IPQ), this project aims to improve dosing accuracy and enable traceable measurements of volume, flow and pressure of existing drug delivery devices and inline sensors operating at very low flow rates (lower than 100nl/min). This can be achieved through the development of new calibration methods and improved metrological infrastructures.
Calibration methods
Regular calibration and maintenance of infusion pumps enables the identification of any equipment issues and ensures the correct dosage is delivered to patients, thus minimising potential safety risks.
A common calibration method to determine the flow rate error of an infusion pump involves the use of an infusion device pump analyser, which gives information on the flow rate, volume and pressure. The analyser functions as a master calibrator to quickly test infusion pump performance; however, it is important that it is calibrated regularly. This method is used in hospital facilities.


Figure 1: Calibration of a syringe pump using the gravimetric method.
The gravimetric method is used extensively in the laboratory by the National Metrology Institutes (NMIs) and accredited laboratories, as a very accurate way to calibrate pumps and flow meters (Figure 1). This method uses a balance to weigh the mass of liquid (ie, water, the specification of water is defined in ISO 3696)5 that is delivered by the pump into a weighing vessel on the balance. As the density of the water is known at the temperature of the test (usually 20°C), this is used to calculate the volume of liquid delivered (volume = mass/density). The volumetric flow rate is determined from the quotient of the total liquid volume and the time taken for the delivery of that liquid.
The volumetric flow rate (Q) can be expressed as:
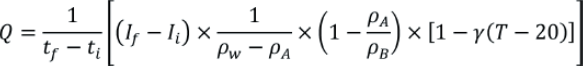
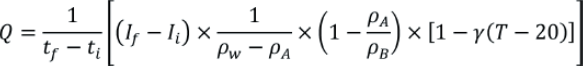
Where tf is the final time, ti is the initial time, If is the final balance reading, Ii is the initial balance reading, ρw is the density of water used for the calibration, ρA is the density of air, ρB is the density of the mass standards used to calibrate the balance, γ is the coefficient of thermal expansion of the disposable syringe and T is the temperature of the water used. More information on the expansion coefficients is provided in ISO 4787.6 It is recommended that additional correction factors be used, including a correction for the evaporation of water during the calibration and a correction for the buoyancy effect of the needle immersed in the water in the beaker.
There are several influence factors that should be considered in a gravimetric calibration:
- Air bubbles – where a sufficient volume of water should be passed through the piping to purge any air bubbles
- Flow stability and pump response time – there is a need to wait until the target infusion flow rate has been reached, as large errors will occur before this time
- The time taken before the required target flow rate is achieved – often referred to as the start‑up delay or delayed onset and depends on the flow rate, type of pump and disposables used
- Incorrect syringe diameter – can lead to large dosing errors; eg, five percent error in the syringe diameter can lead to a 10 percent error in the delivered drug flow rate. Different syringe models and brands can have different diameters, so it is always important to check this.
- Water evaporation – if not controlled properly this can cause an underestimation of flow rate measurements
- Equipment compliance – there are expansion effects in the components owing to the elasticity of the materials, eg, the expansion of a plastic syringe when the pressure increases during the start-up of the pump. Expansion of components can also occur when the flow rate is increased; this increase in the pressure can cause the infusion line, syringe and other equipment to expand. The expansion results in the internal volume increasing hence can cause a delayed response for flow rate changes.7
The gravimetric calibration method is limited when trying to measure ultra-low flow rates, as the measurement uncertainty of this method increases substantially and evaporation becomes a critical error factor. Special set-ups for gravimetric calibrations have been performed by NMIs down to 6μl/h (100nl/min).8 However, new developments in pumping technology, eg, insulin pumps, can deliver extremely low flow rates, typically 10μl/h and much lower. To test these new devices, novel calibration techniques are being developed by NMIs that are capable of measuring flow rates down to 5nl/min. Some of these new calibration techniques are based on optical measurements or displacement methods, eg, following the displacement of the syringe piston over time with an interferometer (Figure 2).9
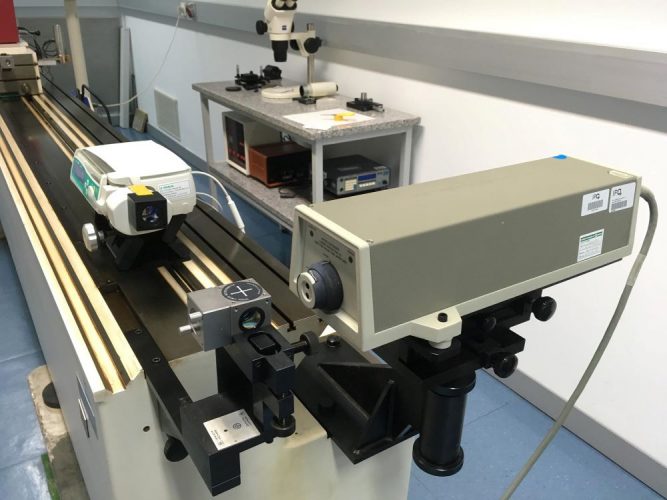

Figure 2: Calibration of a syringe pump using the interferometric method.
Metrologic conformity
The assessment of the performance of a pump being calibrated cannot be entirely based on the determination of the error, even if it is found to be within ±2 percent, which is normally stated by the manufacturer as the maximum permissible error. The uncertainty of the calibration must also be considered in assessing the metrological conformity.
The relative flow rate error of a pump is given by:
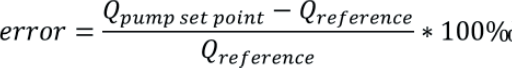
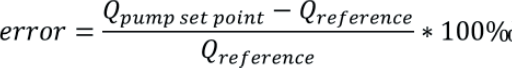
Where Qpump set point is the target flow rate set on the pump and Qreference is the actual flow rate determined in the calibration. A negative error means the pump is delivering over its target flow rate and a positive error means it is delivering less fluid than desired. This definition of error complies with metrological standards.10 However, it should be noted that a different definition is used in medical standards, such as in IEC 60601-2-24:201,11 where the error is given by:
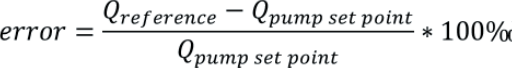
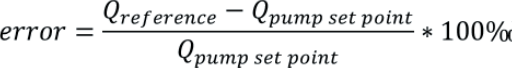
It is important to identify how the error is calculated to avoid false corrections being applied. More detailed information on these conflicting definitions is provided.12
Conclusion
This work highlights the importance of the regular calibration of medical infusion pumps to reduce safety risks by identifying any performance issues and ensuring patients are given the correct dose. The gravimetric method is the most common method used for assessing the performance of an infusion pump analyser; however, other methods are under development for measurement of very low flow rates. The performance analysis of the pump should be reported according to the appropriate references to avoid false corrections. Improving the accuracy of medical devices will reduce dosing errors, meaning that lives can be saved.
About the authors


Elsa Batista has a Masters in Analytical Chemistry from the Universidade de Lisboa/Faculdade de Ciências (UL/FC) and is currently doing a PhD in Mechanical Engineering at Faculdade de Ciências e Tecnologia/ Universidade Nova de Lisboa (FCT/UNL). She started working at the Portuguese Institute for Quality (IPQ) in 1999 and has been responsible for the Volume and Flow Laboratory of the IPQ since 2002. Elsa is the national contact person for EURAMET TCF and is convenor of the Volume sub-group. She is the Co-ordinator of the EURAMET international projects: “Metrology for drug delivery II” and “Establishing metrology standards in microfluidic devices”.


Emmelyn Graham has a Masters in Chemistry and a PhD in advanced optical imaging techniques for microfluidic systems from the University of Edinburgh. She started working at TÜV SÜD National Engineering Laboratory in 2007 in the area of microfluidics, and then specialised in multiphase flow measurement for the oil and gas industry. More recently, she has applied her flow metrology expertise to developing measurement infrastructure for drug delivery applications and microfluidic systems.
References
- US Food and Drug Administration website accessed 26th March 2020 https://www.fda.gov/medical-devices/general-hospital-devices…
- Lucas P, Snijder RA, Timmerman AMDE, et a 2015. Best Practice Guide, EMPIR Metrology for Drug Delivery, Version 13-05-2015
- Rigel Medical (UK), An introduction to infusion pump testing, Version 1.0 – 2014
- Batista E, Furtado A, Pereira J, et al. New EMPIR project – Metrology for Drug Delivery. Flow Measurement and Instrumentation. 2020;72:101716.
- ISO 3696:1987, Water for analytical laboratory use – specification and test methods, 1987
- ISO 4787:2010, Laboratory glassware — Volumetric instruments — Methods for testing of capacity and for use, 2010
- Batista E, Sousa JA, Martins RF. Calibration of Insulin Pumps, Journal of Diabetes and Treatment, Vol 4, Issue 03, 2019
- MeDDII GOOD PRACTICE GUIDE: Calibration of Medical Infusion Pumps, https://drugmetrology.com/wp-content/uploads/2021/05/MeDD_II…
- Batista E, Godinho I, Martins R, et al. (2020) Development of an experimental setup for microflow measurement using interferometry, Flow Measurement and Instrumentation, vol. 75
- JCGM 200:2012, International vocabulary of metrology – Basic and general concepts and associated terms (VIM), 3rd edition, 2012
- IEC 60601-2-24:2019, Medical electrical equipment, Part 2-24: Particular requirements for the basic safety and essential performance of infusion pumps and controllers, 2019
- MeDDII report, Measurement error: two opposite definitions in metrology standards and medical standards, 4/2021 [Internet]. 2021 [cited 13 July 2021]. Available from: https://drugmetrology.com/wp-content/uploads/2021/04/Measurement-error-defenitions… MeDDII has received funding from the EMPIR programme, co-financed by the Participating States and the European Union’s Horizon 2020 research and innovation programme









