COVID-19 antibody therapeutics – where are they now?
Posted: 17 June 2021 | Hannah Balfour (European Pharmaceutical Review) | No comments yet
In this article, EPR’s Hannah Balfour explores the current usage of monoclonal antibody therapies for COVID-19, with commentary from Professor Adrian Streinu-Cercel of the Carol Davila University of Medicine and Pharmacy.

The severe acute respiratory syndrome 2 coronavirus (SARS-CoV-2) swept across the world in 2020, resulting in widespread lockdowns, social distancing regulations and extraordinary suffering. In addition, the novel coronavirus disease 2019 (COVID-19) – caused by SARS-CoV-2 – sparked vaccine, therapeutics and diagnostics races that resulted in the first vaccine, based on a novel technology, being approved in the UK, US and EU in December 2020. This was just 12 months after SARS-CoV-2 was first identified and its genetic sequence released.1 Since then, 14 other COVID-19 vaccines employing various technologies have been approved or authorised for emergency use in numerous countries and 60 more are in development.2
However, though many heralded vaccine approvals as the end to the pandemic, as of 7 June 2021 only 11.8 percent of the global population had received their first vaccine dose and less than five percent had received two doses.3 Much of this is due to inequitable vaccine distribution and that a vaccine has yet to be approved for use in children; though Pfizer and BioNTech’s vaccine recently received US Food and Drug Administration (FDA) and European Medicines Agency (EMA) approval for use in adolescents aged 12 and over.4,5 In the absence of widespread vaccination for most countries, other preventative measures and treatments for COVID-19 are vital to stop deaths and reduce the burden on overstretched healthcare systems.
One type of intervention that has shown promise as a therapeutic for COVID-19 is monoclonal antibodies (mAbs). When interviewed about the use of mAb therapies for treating COVID-19, Adrian Streinu-Cercel, MD, PhD, Professor of Infectious Diseases at the Carol Davila University of Medicine and Pharmacy in Romania and Global Investigator on trials assessing Celltrion’s regdanvimab (CT-P59) mAb therapy, explained: “A vaccine will train the immune system to battle future infections, while an antibody treatment can immediately treat an existing SARS-CoV-2 infection. These powerful tools will complement each other to ensure that the majority of the population is protected from the potentially severe effects of COVID-19.”
Why use mAbs as a treatment for COVID-19?
Professor Streinu-Cercel explained that the primary benefits of mAbs are their specificity and thus safety: “mAbs are laboratory-made proteins that mimic the immune system’s ability to fight off harmful pathogens, such as viruses, and have an advantage over other types of treatment for infection because they are created to specifically target an essential part of the virus. A mAb is created by exposing a white blood cell to a particular viral protein; this white blood cell is then cloned to mass produce antibodies to target that virus.” He added that they are one of the most promising classes of therapies owing to their longstanding track record of safety in humans, their exceptional specificity to the virus (which minimises the risk of off-target effects) and their ability to co-ordinate the immune defence in the fight against infection. In a disease such as COVID-19, where the severest symptoms are in part caused by excessive inflammation and a hyperactive immune response, this specificity is key to ensuring the therapies benefit those with the disease, rather than exacerbating symptoms.
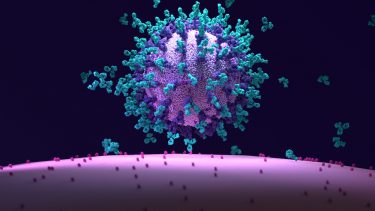

Three dimensional (3D) illustration of monoclonal antibodies bound to SARS-CoV-2 Spike proteins and neutralising the virus – preventing it from entering human cells through the interaction with the ACE2 receptor.
In most cases, mAbs designed to treat COVID-19 target the SARS-CoV-2 Spike (S) protein, a glycoprotein expressed on the surface of the virus which is required for it to infect human cells. SARS-CoV-2 infects cells via interaction between the receptor binding domains (RBDs) on the S protein and the angiotensin-converting enzyme 2 (ACE2), which acts as a receptor on human cells. By inhibiting this interaction, mAbs aim to combat the spread of the virus through the tissues of the body, thus reducing the severity of symptoms from infection. They also have immunomodulatory capabilities which can assist the immune system in fighting off the infection.
“While new research has shown many COVID-19 patients are asymptomatic or recover from mild forms of the disease, high-risk patients are more likely to progress to severe disease requiring hospitalisation, oxygen therapy and even risk of death.6 mAb treatment, when given at an early stage, can reduce the progression rate to severe COVID-19 in mild-to-moderate patients,” stated Professor Streinu-Cercel. In this way, he continued, they could alleviate the burden on healthcare systems by enabling at-risk outpatients to avoid hospitalisation. Furthermore, anti-Spike protein mAbs represent the only specific treatment against SARS-CoV-2 currently available.

He added that therapeutic mAbs have shown to be more effective when administered early in various respiratory infections including influenza, Severe Acute Respiratory Syndrome (SARS), Middle East Respiratory Syndrome (MERS) and Ebola. Not only do they reduce viral load by inhibiting viral entry into cells, said Professor Streinu-Cercel, but they can also act as immune rate limiting agents, dampening down the hyperactive immune response that may be causing damage to tissues. Both are key roles in reducing morbidity and mortality. Some of the key mAbs currently in development include tocilizumab, sarilumab and siltuximab, which are all interleukin 6 (IL-6) inhibitors being assessed for their ability to combat cytokine storms7 – the release of a large amount of pro-inflammatory factors called cytokines into the blood. Cytokine storms are believed to be the cause of immune-mediated tissue damage and the severest COVID-19 manifestations, including Acute Respiratory Distress (ARDS) and organ failure. Other immunomodulatory targets include, but are in no way limited to, granulocyte-macrophage colony-stimulating factor (GM-CSF), the CCR5 chemokine receptor, IL-1β and interferon gamma (IFNɣ) and ongoing research is assessing their role in the clinic.7
In addition, Professor Streinu-Cercel explained that monoclonal antibodies can complement vaccines in another way: by offering rapid protection against infection, especially in those who may be immunocompromised or have a weakened immune system and are not eligible for vaccines – or may not be fully protected by them as a result.
But why use mAbs, which are more costly and difficult to produce and store, instead of a small molecule drug?
Professor Streinu-Cercel stated that despite small molecule drugs being convenient – as they can often be more easily manufactured, stored, distributed and administered to patients in the form of a pill – they can easily penetrate tissues and may not be as specific as mAbs, so could bind to many human proteins, causing off-target effects. As a result of their size, large biological drug molecules, such as mAbs and naturally occurring antibodies, do not penetrate tissue very well and are highly specific for a single target so rarely cause undesirable side effects. Their size is also a limitation though, as it necessitates intravenous administration in a clinical setting.
Current state of mAb therapies for COVID-19
According to a study published in April 2021, there are currently over 50 mAbs targeting SARS-CoV-2 in various stages of development.8 As of 1 June 2021, there are four mAbs (etesevimab, casirivimab, imdevimab and sotrovimab) and two mAb combinations (bamlanivimab plus etesevimab and casirivimab plus imdevimab [known as REGN-COV2]) approved by the FDA for emergency use in the treatment of mild to moderate COVID-19 in non-hospitalised patients with laboratory confirmed SARS-CoV-2 infection who are at high risk for progressing to severe disease and/or hospitalisation.8 Bamlanivimab alone was originally available as a single agent; however, the emergence of SARS-CoV-2 variants that are resistant to it resulted in the FDA revoking its single agent emergency use authorisation in April 2021.
mAb therapies have demonstrated their ability to safely prevent the development of severe and critical COVID-19 symptoms when administered early in the course of infection”
The EMA’s Committee for Medicinal Products for Human Use (CHMP) is currently undertaking rolling reviews on the data for bamlanivimab plus etesevimab, regdanvimab, REGN-COV2 and sotrovimab.9 In each case, they can be used in the European Union (EU) to treat COVID-19 in patients who do not require supplemental oxygen and who are at high risk of their COVID-19 disease becoming severe. Sotrovimab, bamlanivimab plus etesevimab and bamlanivimab alone can be used in patients aged 12 years and over, while regdanvimab is only recommended for use in adults. Each was found to reduce the likelihood of hospitalisation or death in at-risk outpatients with mild-to-moderate COVID-19 symptoms.
The combination of bamlanivimab plus etesevimab was recommended by the EMA for use based on Phase II/III data, in which treatment with the combination significantly decreased (by -0.57) SARS-CoV-2 log viral load at day 11 compared with placebo. Additionally, the proportion of patients with COVID-19–related hospitalisations or emergency department visits was 5.8 percent for placebo (nine events) and 0.9 percent (one event) for the combination treatment.10
REGEN-COV was recommended by the CHMP for use based on a study that investigated the effects of the combination in outpatients with COVID-19 who do not need supplemental oxygen. The interim data from 275 patients in the study showed the percentage of patients reporting at least medically attended visits were six for placebo and three for REGEN-COV and in the subgroups that were SARS-CoV-2 serum antibody–negative at baseline these were 15 and six percent respectively.11
Regdanvimab’s emergency use authorisation was based on results from Study CT-P59 3.2 Part 1, which is a randomised, double-blind, placebo-controlled trial where patients were randomised 1:1:1 to receive regdanvimab at doses of 40mg/kg (n=105) or 80mg/kg (n=111) or placebo (n=111). In the trial, only nine of the 204 patients (4.4 percent) receiving doses of regdanvimab required hospitalisation, oxygen therapy or experienced mortality, compared to nine of the 103 patients (8.7 percent) receiving placebo.12
The emergency use of sotrovimab was based on Phase II/III COMET-ICE trial results, which showed progression of COVID-19 resulting in hospitalisation at day 29 was reduced by 85 percent in the group who received a single 500mg infusion of sotrovimab (n=291), compared to placebo (n=292, the primary endpoint). Additionally, only two patients receiving sotrovimab (less than one percent) developed severe or critical COVID-19 symptoms, compared to 19 of the placebo patients (seven percent). There were no deaths in the sotrovimab arm and one in the placebo arm.13
Therapeutic antibodies and viral variants
Recently, studies have shown that certain mAb therapies, such as bamlanivimab alone (mentioned above), may be rendered less effective as a result of emerging SARS-CoV-2 viral variants harbouring mutations in their S protein. Commenting on the issue, Professor Streinu-Cercel said: “Certain mutations in the SARS-CoV-2 S protein not only appear to have increased its ability to bind to ACE2 on human cells but variants carrying this mutation have also shown some resistance to antibodies taken from patients who have recovered from the infection.
updated combinations, or “cocktails”, of mAbs that target areas of the virus that change less frequently showed promise in neutralising the latest virus variants”
“Numerous therapeutics have been developed to treat COVID-19; however, many are based on initial variants of SARS-CoV-2 and may be less effective against recent ones. As the virus has been spreading, it has developed mutations, which in some cases can make the virus more infectious. In laboratory tests, a team of researchers analysed the efficacy of a range of COVID-19 antibody-based therapeutics against the latest variants of SARS-CoV-2. They noted that some combinations of monoclonal antibody therapeutics were less effective against these new variants. However, they also found that other updated combinations, or “cocktails”, of mAbs that target areas of the virus that change less frequently showed promise in neutralising the latest virus variants.”14,15
Conclusion
Overall, mAb therapies have demonstrated their ability to safely prevent the development of severe and critical COVID-19 symptoms when administered early in the course of infection. Several have been authorised for emergency use; however, resistance is emerging and must be monitored closely to ensure that therapies continue to be efficacious as the pandemic evolves. With more than 50 mAbs, aimed at a range of targets, both SARS-CoV-2 and human, currently under development and testing for COVID-19 indications, their potential to continue to treat and prevent COVID-19 seems assured.
References
- Zheng J. SARS-CoV-2: An Emerging Coronavirus that Causes a Global Threat [Internet]. International Journal of Biological Sciences. 2020 [cited 20 May 2021];16(10):1678-1685. Available from: https://dx.doi.org/10.7150%2Fijbs.45053
- Craven J. COVID-19 vaccine tracker [Internet]. Raps.org. 2021 [cited 20 May 2021]. Available from: https://www.raps.org/news-and-articles/news-articles/2020/3/covid-19-vaccine-tracker
- Ritchie H, Ortiz-Ospina E, Beltekian D, et al. Coronavirus (COVID-19) Vaccinations – Statistics and Research [Internet]. Our World in Data. 2021 [cited 8 June 2021]. Available from: https://ourworldindata.org/covid-vaccinations?country=OWID_WRL
- Coronavirus (COVID-19) Update: FDA Authorizes Pfizer-BioNTech COVID-19 Vaccine for Emergency Use in Adolescents in Another Important Action in Fight Against Pandemic [Internet]. US Food and Drug Administration. 2021 [cited 20 May 2021]. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update…
- First COVID-19 vaccine approved for children aged 12 to 15 in EU – European Medicines Agency [Internet]. European Medicines Agency. 2021 [cited 28 May 2021]. Available from: https://www.ema.europa.eu/en/news/first-covid-19-vaccine-approved-children-aged-12-15-eu
- Fallon P. Most COVID-19 Cases Caused By People Without Symptoms. Healthline, 2021. Available at: https://www.healthline.com/health-news/most-covid-19-cases-come-from-people-without-symptoms.
- Anti-SARS-CoV-2 Monoclonal Antibodies | COVID-19 Treatment Guidelines [Internet]. National Institutes of Health. 2021 [cited 28 May 2021]. Available from: https://www.covid19treatmentguidelines.nih.gov/…
- Deb P, Molla M, Saif-Ur-Rahman K. An update to monoclonal antibody as therapeutic option against COVID-19. Biosafety and Health [Internet]. 2021 [cited 28 May 2021];3(2):87-91. Available from: https://doi.org/10.1016/j.bsheal.2021.02.001
- COVID-19 treatments: under evaluation – European Medicines Agency [Internet]. European Medicines Agency. 2021 [cited 28 May 2021]. Available from: https://www.ema.europa.eu/en/human-regulatory/overview/…
- Gottlieb R, Nirula A, Chen P, et al. Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19[Internet]. JAMA. 2021 [cited 28 May 2021];325(7):632. Available from: https://jamanetwork.com/journals/jama/fullarticle/2775647
- Weinreich D, Sivapalasingam S, Norton T, et al. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19 [Internet]. New England Journal of Medicine. 2021 [cited 8 June 2021];384(3):238-251. Available from: https://www.nejm.org/doi/full/10.1056/NEJMoa2035002
- ANNEX 1 CONDITIONS OF USE, CONDITIONS FOR DISTRIBUTION AND PATIENTS TARGETED AND CONDITIONS FOR SAFETY MONITORING ADRESSED TO MEMBER STATES FOR UNAUTHORISED PRODUCT Regkirona (regdanvimab) [Internet]. European Medicines Agency; 2021 [cited 8 June 2021]. Available from: https://www.ema.europa.eu/en/documents/referral/celltrion-use-regdanvimab…
- ANNEX 1 CONDITIONS OF USE, CONDITIONS FOR DISTRIBUTION AND PATIENTS TARGETED AND CONDITIONS FOR SAFETY MONITORING ADDRESSED TO MEMBER STATES FOR UNAUTHORISED PRODUCT SOTROVIMAB [Internet]. European Medicines Agency; 2021 [cited 8 June 2021]. Available from: https://www.ema.europa.eu/en/documents/referral/sotrovimab…
- Huzar T. Antibody “cocktails” effective against SARS-CoV-2 variants [Internet]. Medical News Today. 2021. [cited 31 March 2021]. Available at: https://www.medicalnewstoday.com/articles/antibody-cocktails…
- Chen RE, Zhang X, Case JB, et al. Resistance of SARS-CoV-2 variants to neutralisation by monoclonal and serum-derived polyclonal antibodies. Nature Medicine. 2021. [Cited 31 March 2021]. Available at: https://www.nature.com/articles/s41591-021-01294-w
Related topics
Antibodies, Biologics, Clinical Trials, Drug Development, Drug Safety, Drug Targets, Immunotherapy, Industry Insight, Research & Development (R&D), Therapeutics, Vaccines, Viruses
Related organisations
BioNTech, Carol Davila University of Medicine and Pharmacy, Pfizer, The European Medicines Agency (EMA), US Food and Drug Administration (FDA)
Related drugs
Casirivimab, etesevimab, Imdevimab, Kevzara (sarilumab), Regdanvimab (CT-P59), REGEN-COV™ (casirivimab and imdevimab antibody cocktail), RoActemra (tocilizumab), siltuximab (SYLVANT®), Sotrovimab (VIR-7831)
Related people
Related diseases & conditions
Acute Respiratory Distress Syndrome (ARDS), Coronavirus, Covid-19, Cytokine storm, Ebola, Influenza, Middle East Respiratory Syndrome (MERS), Severe Acute Respiratory Syndrome (SARS)









