Cleanroom infrastructure innovation to fulfil the needs of cell and gene therapies
Posted: 26 April 2021 | Maik W Jornitz | No comments yet
Biopharmaceuticals have transformed the patient care landscape, but alongside this step change in treatment capability comes the need for a similar paradigm shift in manufacturing capability. Here, Maik W Jornitz, President & CEO of G-CON Manufacturing Inc. highlights how cleanroom infrastructure must develop to keep pace with ever evolving industry needs.


Processing space evolutions
Some 40 years ago, when biologics entered the therapeutic market, process and facility designs required a major shift. Where small molecule therapeutics had only a few process unit operations, the biologics manufacturing process has a multitude of complex process unit operations, some of which require strict segregation. This processing shift necessitated an entirely new layout with regard to the process, facility and segregation, such as up- and down-stream or pre- and post-viral. The complexity of the bioprocess ultimately required new consideration of the facility designs. These designs were unique and not necessarily comparable to small molecule processes. This meant that entirely new facilities had to be built – and fast. The capital investments made were substantial and risky, since the investment decision had to be made early in the drug product development process, as the sites typically took three to four years to build. The typical biologics site used about four to six 10-15,000L bioreactors and required large-scale equipment units downstream of the reactors. Everything was big and everything was stainless steel, with the respective complexity in setup and cleaning. The reason for the large process scales was the lower yield results from these early day expression systems.
The capital investments made were substantial and risky
With the expression rates and yields improving, the up-stream bioreactor volumes dropped to a scale where stainless steel process equipment converted to single-use processes. These advantages have been described in many publications, not just from a total cost ownership reward, but also from a utility standpoint – with the reduced needs of water and steam.1-3 The setup and turnover of single-use bioprocesses are much faster with resulting efficiency gains. In addition, these functionally closed processes could potentially accommodate multi product processes. The phrase “flexible facility” was used, or better abused, to describe a facility with flexible single-use processes;4 albeit nothing is flexible within a facility when only the process is flexible. The flexibility emphasis had to be adjusted to clearly delineate the process from the facility; both being essential, not just one.
The fact that flexibility in its entirety is needed became very clear when the industry, once again, changed its therapeutic modality to new treatments: cell and gene therapies.5 In particular, cell therapy – a patient-based therapeutic entity – requires a high level of flexibility in both the process and facility; thus recognising what a truly flexible facility needs to achieve. The processes use single-use equipment as much as possible, since the process must be strictly contained. The patient is the batch and any process downfall would be detrimental. This also meant that the containment requirement was no longer just for the process but encompassed the next layer: the cleanroom infrastructure. It is necessary to create flexibility on an infrastructure level, to encompass the capability to scale-out without interruption, potentially move cleanroom infrastructure to a centralised or decentralised location and configure the facility with the growth of the process and patient base. The rapid rise of cell and gene therapies required a necessary paradigm shift in the vision, design, build and installation of cleanroom infrastructures. Flexible facilities are now finally becoming what they should have been for years: entirely flexible and quickly deployed infrastructure systems.
The request for higher flexibility and agility, besides rapid deployment and shorter time to run, has generated positive reception and adoption of innovations within the manufacturing area designs and cleanroom infrastructures. The shift to new, smaller volume therapies also required a shift to new facility solutions. The obsolete, traditional arrangements are being replaced by more robust design and material options. Modular and off-site prefabricated podular, cell and gene therapy cleanroom infrastructures are being adopted to gain the required flexibility and shorter time-to-run. For industry’s benefit, modular and podular designs and materials have evolved further, creating an enhanced toolbox of facility design choices. This evolution has not stopped at the materials and construction of such cleanroom infrastructures, but has extended to standardisation of designs and turnkey solutions to reduce design timelines even further. A cleanroom for cell therapy applications is a confined and not necessarily highly variable space, so why not mass produce such space as in the automobile industry and bring the costs and delivery time down? These possibilities exist with off-site, prefabricated podular cleanroom units.
The need is speed of capacity implementation
Appraising the capacity demands of cell and gene therapies, it is clear that previous facility designs and construction modes cannot fulfill the demand. Demand curves showed that the ramp up needs to be much faster to be able to serve the indications in the clinical pipeline. However, traditional cleanroom infrastructures do not meet the delivery speed and reliability criteria. Both stick‑built7 and modular-built8 cleanroom infrastructures are fixed installed on-site, encountering the same obstacles as onsite‑built, including reduced productivities, varying work forces and trades, transfer to the construction area, head count density, lay‑down areas and supply flows, to name a few. Moreover, once the cleanroom infrastructure is finalised, the often interconnected, central HVAC (heating, ventilation and air conditioning) system does not allow uninterrupted scale-out. Furthermore, mezzanine levels and structural fixations do not allow fast deployable high span shell buildings. The built-in-place cleanroom infrastructures have no flexibility to be either moved or amended, which can be a financial burden, as an initial clinical material process cannot be converted to a commercial scale or moved to the commercial scale building. This functionality is important to enable cleanroom infrastructure flexibility.
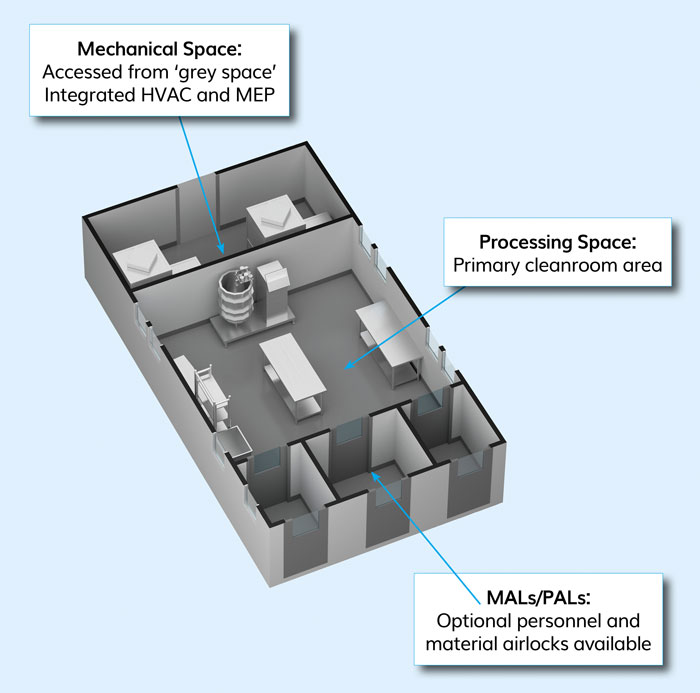

Figure 1: Autonomous podular cleanroom unit (courtesy G-CON Manufacturing Inc)
Scaling without interrupting the existing processes became a major issue for a cancer vaccine company in the mid-2000s. The challenge posed was to design a cleanroom unit that was autonomous – ie, included the full functionality of HVAC, fire suppression, automation, utility connections, etc – and could be off-site built, moved and prequalified. Such a cleanroom unit would allow it to scale-up the necessary processing space when needed, without large upfront investments and interruption of the existing processes. The cleanroom infrastructure that was developed as a result are off-site, prefabricated podular cleanroom systems (Figure 1).
These units can be used as cleanroom blocks, either being interconnected to a larger cleanroom cluster or as individual units placed side by side (Figure 2). The latter is especially useful for cell therapy applications, as these units act as a walkable isolator, with the HVAC system in the mechanical space and the duct installed in interstitial space of the cleanroom box. These units are fully functional and prequalified before they leave the manufacturing site, so the end user can be assured it works when it reaches the site. The concept is comparable to a car being predesigned, prefabricated and prequalified, thus preventing time and money being wasted on the design and being sure the technology works. The podular units are installed into a shell building, preferably a high span warehouse-type shell, thus being termed “box-in-box”. It differs from the container-based, total facility infrastructure, like Pharmadule or KuBio sites, which are interconnected to an outside facility and, again, once built cannot be moved or changed.


Figure 2: Side by side cleanroom POD units with uninterrupted scalability (courtesy G-CON Manufacturing Inc)
An added beneficial factor with the flexibility of off-site construction of podular cleanroom units, is the fact that off-site construction creates the flexibility of headcount numbers or productivity adjustments.6 This means the production timelines and costs can be well defined and reliably held in off-site construction. It has been stated that this is not the case in the on-site, traditional built projects, which experience undesirable cost and delivery time overruns, which can range from 20-30 percent. What looked like an attractive project pricing and execution timeline, can quickly become a project management nightmare.
Furthermore, the productivity in an off-site build is typically higher; the same team builds the units in a well-known and specified construction area. This means that the construction speed is higher and in conjunction with the parallel-built shell building, utilities, auxiliary infrastructure and process equipment a facility can be completed in months and not years. Examples of the time value have been reported and fast track projects are facilitated in four to eight months, depending on the size.
With the rising growth of new therapies and indications, the demand for capacity is increasing rapidly, thus requiring facilities to be built much faster to meet the demand. This demand is not expected to subside in the foreseeable future and a CAGR of 30 percent plus has been mentioned. This puts further pressure on faster facility construction, which requires us to learn from other industries.
Standardisation is key for true acceleration
The consumer goods and semiconductor industries revealed that facility construction speed and also manufacturing flexibilities require standardisation. Without this, projects require lengthy design phases, thousands of man hours, back and forth reiterations, the construction of something unknown, with unknown labour details and varying quality, etc. These two cited industries work in highly competitive pricing environments, thus their manufacturing efficiencies have to be unconditionally optimised and require exceptional flexibility. These efficiencies and flexibilities can only be achieved by standardising the manufacturing modules within a set shell building footprint. The manufacturing equipment gets standardised right from the start of a new product development and typically is cloneable to scale either within the current manufacturing footprint or with the desire of global spread. These cloneable manufacturing modules will have set dimensions, as these standard dimensional units must fit into a standard dimensional shell building like pieces of a puzzle. The examples also demonstrated the capability for a manufacturing module to be removed from a shell building and replaced by a module with a higher demand in a particular region, thus representing flexibility. The entire site is standardised: equipment, manufacturing module, utilities and shell building. When a new site is built, everybody knows what to expect. Another key benefit is that the staff of a new site can be trained in an existing one, in advance. This model allows maximum efficiencies and flexibilities. Moreover, a site does not need to be gutted if a product lifecycle runs out, but just manufacturing modules exchanged for a new product to be supplied.
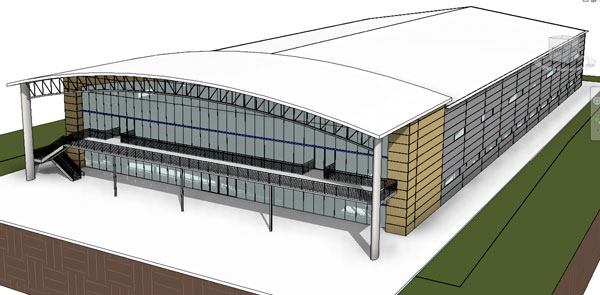

Figure 3: iCON, 4 x 2,000L mAb facility (courtesy Integrated Project Services, LLC (IPS))
There is no reason why this could not happen in the biopharmaceutical industry. The off‑site, prefabricated cleanroom units, as well as standardised single-use process assemblies, are the first step in that direction. Standard cleanroom units are already available and progress has been made on turnkey facilities (Figure 3); both initiatives will support facility choice from a catalogue of standard designs, abbreviating if not eliminating the lengthy and costly design phases. Why reinvent the wheel or design a new, unknown entity, when standard turnkey solutions would allow the building or cloning of an infrastructure that has already been built and validated? This would not only enhance scaling effect and lower costs, but also enhance the reliability of capacity planning. Other industries have realised these financial, planning and project management benefits and the biopharmaceutical industry can benefit from their success.
In summary
New facility and cleanroom infrastructure needs for the rapidly rising demand of the cell and gene therapy capacity have been voiced. For example, the Biophorum Modular & Mobile Technology Roadmap presented definite industry needs which are required to supply the facilities of the future. These needs must be addressed, as the current and near future capacity demand cannot be fulfilled.
The stated needs are:
- Reliability of the delivery time and cost budget for a new infrastructure and capacity build-out, in order to plan appropriately
- Consistent construction quality and assured full functionality
- Facilities and cleanroom infrastructures must be rapidly deployable with construction, installation and qualification timelines of four to eight months, not years
- Scaling of the facility should not disrupt existing processes, which means capital investment decisions can be delayed and/or made gradually
- Cleanroom infrastructures need to be repurposable, rather than being sunk assets
- Cloning of facilities and infrastructures help design timeline abbreviation, personnel training and regulatory familiarity
- Mobility of the infrastructures helps to move capacities where they are required or allow temporary manufacturing in a location to be moved, at a later stage, to the final location.
The biopharmaceutical industry realised a quantum leap in technologies with single-use processes. However, new technology implementation cannot stop at the process level, but requires to be extended to the next: the cleanroom infrastructure facility. Other industries have demonstrated the capability for innovative solutions. These solutions are available but require a mindset of change and motivation to implement them.
Maik W Jornitz
Maik W Jornitz, President and CEO of G-CON Manufacturing Inc., is a technical expert with over 30 years of experience in bioprocesses, especially sterilising grade filtration and single-use technologies, including regulatory requirements, integrity testing, systems design and optimisation. Maik has published 11 books, 18 book chapters and over 100 scientific papers. He is the former Chair of the PDA Board of Directors and Science Advisory Board, and member of multiple PDA Task Forces. He is a working member of Biophorum, ASTM, an advisory board member of the Biotechnology Industry Council, ICAV and multiple science journals. He has recently been recognised as one of the top ten global industry influencers. As a faculty member of various training activities, including PDA TRI, he trains members of the industry and regulatory authorities on a frequent basis. He received his M.Eng. in Bioengineering at the University of Applied Sciences in Hamburg, Germany and accomplished the PED programme at IMD Business School in Lausanne, Switzerland.
References
1. Sinclair A, Monge M. (2002) Quantitative economic evaluation of single use disposables in bioprocessing. Pharmaceutical Eng 22, 20-34.
2. Priebe PM. (2004), “Advances in Fluid Processing Technologies”, PDA SciTech Conference, Orlando
3. Pralong A. (2013) “Single-use technologies and facility layout – a paradigm shift” Biopharma Asia Magazine, 2 (1), pp.12-14
4. Levine HL, Lilja JE, Stock R, Hummel H, Jones SD. (2012) Efficient, Flexible Facilities for the 21st Century, BioProcess International 10(11)
5. Jornitz M, Backstrom S. (2018) Factors in Cleanroom Decision Making in Contamination Control in Healthcare Product Manufacturing, PDA DHI
6. McKinsey & Company, “The next normal in construction. How disruption is reshaping the world’s largest ecosystem”, June 2020
7. Stick-built typically defined as framed epoxy-coated gypsum wall erected on-site to a fixed structure
8. Modular-built defined as monolithic uPVC-coated honeycomb panels, manufactured off-site and installed on-site to a fixed structure
Issue
Related topics
Biologics, Biopharmaceuticals, Bioprocessing, Bioproduction, Cleanrooms, Gene therapy, Manufacturing, Production, Therapeutics, Vaccines









