Manufacturing of CAR T-cell therapies: an overview
Posted: 18 February 2021 | Maciej Nakoniecznik (University College London) | No comments yet
The emergence of CAR T-cell therapies has ignited a revolution in the field of cancer treatments. While existing products show outstanding curative capability, they are hampered by significant clinical and commercial challenges, many of which stem from their manufacturing process. Maciej Nakoniecznik discusses how a combination of biological and technological advances in the field may allow CAR T cells to reach their full therapeutic potential.
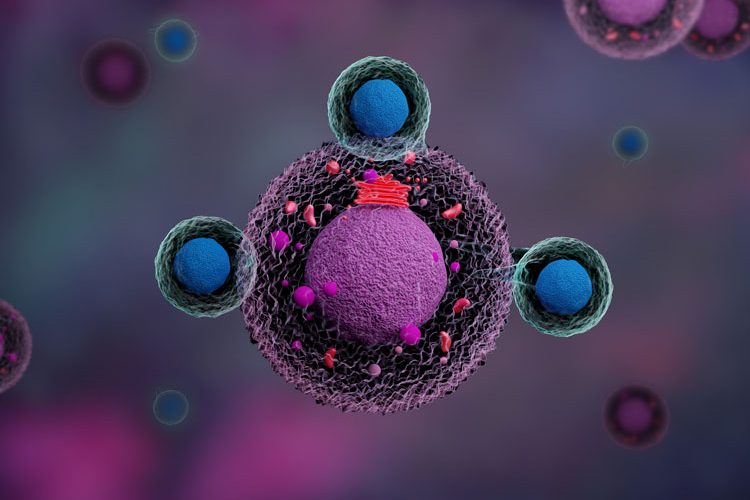

Chimeric antigen receptor (CAR) therapies target cancerous cells by utilising genetically modified T lymphocytes. This approach combines the specificity of a high-affinity, antibody-derived recognition domain with the cytolytic capabilities of T cells. Contrary to their natural mechanism of action, which involves the presentation of the antigen by the major histocompatibility complex (MHC) and the activation of the T-cell receptor (TCR), CAR T cells can recognise and destroy target cells directly.
First-generation CAR constructs comprised three distinct elements: the antigen-binding domain, the transmembrane domain connected to it by the hinge, and the intracellular CD3ζ signalling domain. The following CAR generations contain additional co-stimulatory domains that enhance their clinical efficacy. A range of co-stimulatory domains have been evaluated for use in CAR T cells and their effect on the cells varies from an increase in potency and cytolytic activity to improved in vivo persistence.1
Issue
Related topics
Anti-Cancer Therapeutics, Biopharmaceuticals, Bioprocessing, Bioproduction, t-cells, Therapeutics
Related organisations
European Medicines Agency (EMA), University College London (UCL), US Food and Drug Administration (FDA)









