Fast-scan differential scanning calorimetry
Posted: 2 August 2008 | Simon Gaisford PhD, School of Pharmacy, University of London | No comments yet
Differential scanning calorimetry (DSC) is a widely used technique within the pharmaceutical industry because the range of phase transitions it can measure usually allows near complete physical characterisation of a new active principal early during preformulation. In addition, because DSC measures a property change that is ubiquitous† (heat) there are very few samples that cannot be investigated.
Differential scanning calorimetry (DSC) is a widely used technique within the pharmaceutical industry because the range of phase transitions it can measure usually allows near complete physical characterisation of a new active principal early during preformulation. In addition, because DSC measures a property change that is ubiquitous† (heat) there are very few samples that cannot be investigated.
Differential scanning calorimetry (DSC) is a widely used technique within the pharmaceutical industry because the range of phase transitions it can measure usually allows near complete physical characterisation of a new active principal early during preformulation. In addition, because DSC measures a property change that is ubiquitous† (heat) there are very few samples that cannot be investigated.
Its suitability for polymorph screening is a particularly strong asset. As with all analytical techniques, DSC technology is constantly evolving and improving and three recent derivatives have become popular. These are:
- Temperature-modulated DSC
- High-sensitivity DSC
- Fast-scan DSC‡
Each technique has benefits for certain applications and it is important to understand these in order that the most appropriate analysis strategy is selected for a particular compound and, more importantly, phase transition. Temperature-modulated DSC1 (TM-DSC) is particularly useful pharmaceutically for isolating and quantifying glass transitions while high-sensitivity DSC2,3 (HS-DSC) was developed for studying dilute solutions of macromolecules (usually biologicals). The main benefits of fast-scan DSC (FS-DSC) are simply to increase the size of the measured signal and to reduce the experimental time-frame, which means that it has benefits for virtually all applications of the technique. In addition, fast scan rates can be used to shift the transition temperatures of kinetically-limited events (defined below) which leads to interesting applications of the technique in the pharmaceutical arena. Instruments are commercially available4 that achieve heating rates of up to 500 K/min, while recent developments in DSC technology5 have resulted in an instrument capable of scanning at up to 2000 K/min. The purpose of this article is to discuss the principles underpinning FS-DSC, to review the instrumentation available and to discuss the latest applications of this important analytical tool in the context of physical pharmacy.
Principles of operation
In DSC the heat flow (Φ, in W) from a sample is measured, relative to an inert reference, as that sample is heated or cooled in accordance with an underlying temperature programme. The temperature programme can be linear or modulated by some mathematical function, as noted earlier. DSC data can be presented in a number of ways; most commonly heat flow is plotted versus temperature although it is also common in the biological arena to plot heat capacity versus temperature (obtained by dividing the heat flow data by the heating rate). It is noted that if the area under a peak in a DSC trace is integrated to obtain the total heat output (Q, in J) then the data must be plotted with time (s) on the x-axis, rather than temperature; most DSC software packages automatically take this into account and the user ‘integrates’ the power versus temperature data.
There are two principal types of DSC design; heat-flux and power compensation. In heat-flux DSC, the sample and reference materials are heated or cooled from a common furnace and the temperature difference (ΔT) between the sample and reference is recorded. The heat flow occurring in the sample is proportional to the measured temperature difference and is calculated by multiplying the data by a calibration function.
In power-compensation DSC, the sample and reference materials are heated or cooled from separate furnaces. The instrument varies the power supplied by the two furnaces to maintain a constant temperature difference between the sample and reference and the power difference (ΔP) between the sample and reference is measured directly.
Information from DSC data
Irrespective of the type of DSC used, because the data are differential (i.e. they are normalised to the response of a thermally equivalent, inert reference) if the sample does not undergo a process during the experimental run then the DSC plot should be a horizontal line at y = 0. If the sample and reference have different heat capacities, but the sample still does not undergo any change during the experiment, then the DSC plot will remain a horizontal line but it will be displaced from y = 0 by an amount proportional to the difference in heat capacity between the sample and reference materials. Comparison of the offset value for a sample with similar data recorded for an inert reference material of known heat capacity (such as sapphire) is the basis of heat capacity determination by DSC.
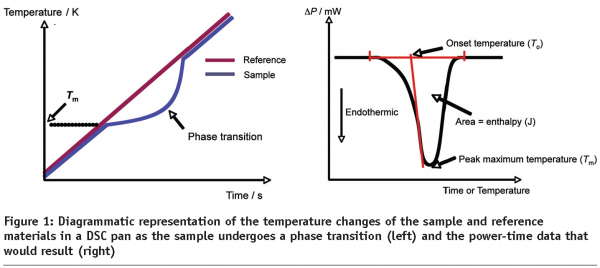

If the sample undergoes some phase transition, then the DSC trace will display a non-baseline signal, the nature of which is governed by the type of event occurring. If the transition is of a simple, one-step nature then a peak will be seen. Analysis of the peak can produce several useful parameters, including the enthalpy of the transition (ΔH), the onset temperature (To) and the peak maximum temperature (Tm), Figure 1. For more complex transitions, such as the melting and subsequent crystallisation of a polymorph, more complex data may be observed. If the sample undergoes a glass transition then a step-change in the baseline will be seen at the glass transition temperature (Tg). Further discussion of these events is given below, but it is sufficient to note here that all of these changes can be used to characterise and identify pharmaceutical materials.
Benefits of fast heating rates
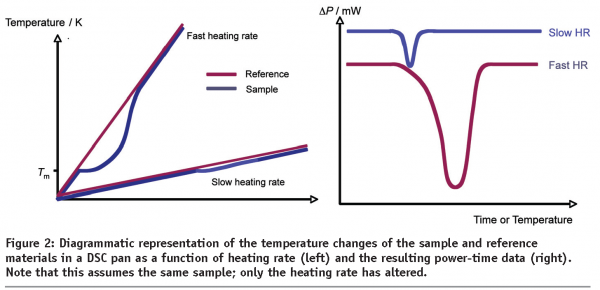
With all DSC instruments there is a compromise between resolution (the separation between thermal events) and sensitivity (the magnitude of each thermal event) that is dependent upon the temperature scan rate. As a general rule, slow scan rates result in good resolution but poor sensitivity while fast scan rates result in poor resolution but good sensitivity. To understand this statement, it is best to consider the processes that occur during a thermal event, such as melting. Figure 2 shows temperature versus time plots for the sample and reference materials respectively as they are heated at different scan rates6. Before the melt, the sample and reference are heated at the same rate and are consequently at the same temperature. As the sample starts to melt, the energy being supplied to it by the furnace is utilised to satisfy the enthalpy of fusion rather than raise temperature and the sample momentarily remains at a constant temperature. The reference, conversely, continues to rise in temperature and so there is a temperature lag between the sample and reference. At slow heating rates, the lag is small and the system returns to equilibrium relatively quickly. The net result of this is a small displacement between the sample and reference and, consequently, a small peak on the DSC thermal curve. When the heating rate is large, similar principles hold; the time taken for the instrument to respond to a change in ΔT is the same, but over time the lag between the sample and reference is greater and the peak on the thermal curve is correspondingly larger.

Fast-scan DSC instrumentation
Operating a DSC at fast-heating rates requires specialised design; specific factors that must be considered include the overriding need to maintain control of temperature, the data capture rate and the effect of time constants. There are two current technologies with which FS-DSC measurements can be made. Either a conventional instrument can be modified to achieve fast heating and cooling rates7 or solid-state ‘chip’ calorimeters can be employed8. The immediate benefit in the former case is one of familiarity and compatibility with the instrument, software and sample handling apparatus. Drawbacks include rather modest heating rates, on the order of a few hundred K/min and, because of the relatively long time constant of a conventional DSC transducer (ca. 2-3 s), a reduced ability to resolve closely occurring events. Solid-state calorimeters can achieve extremely fast heating rates (up to 106 K/s) with very small time constants (on the order of 10-3 s). However, solid-state calorimeters are often very small and usually require samples to be deposited by film casting, frequently onto a thin protective membrane. This means it is difficult to weigh the sample and although they can be cleaned and re-used, solid-state calorimeters cannot at present be considered robust enough for general application outside of a specialised research laboratory.


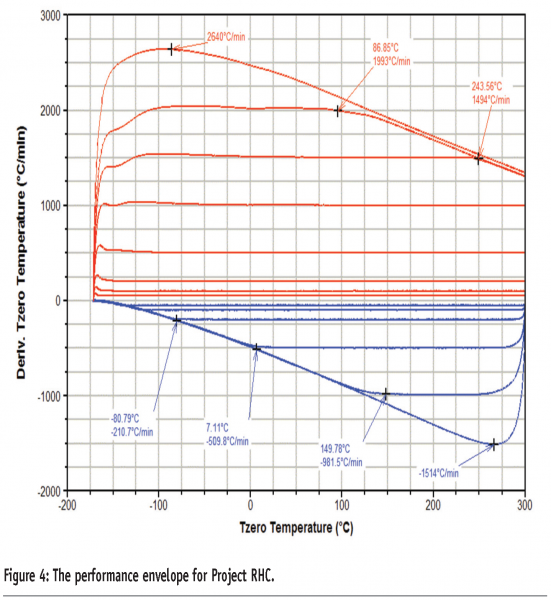
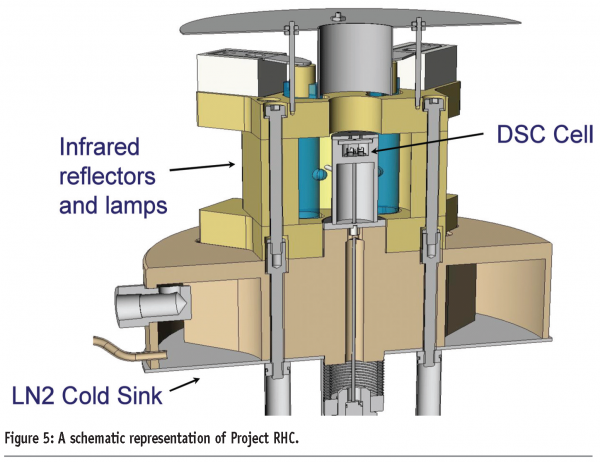
Consideration of ways to ameliorate these design compromises has led to the development of a new design of DSC for achieving fast heating rates, known as Project RHC (TA instruments, USA)5. A number of technological advances have been combined to produce a heat-flux instrument capable of achieving heating rates of up to 2000 K/min. These include reducing the size of the sample, sample pan, sensor and enclosure (the sample pan is less than 1.6 mm in diameter, Figure 3, and the total mass of the measurement cell is less than 25 g) and incorporating infrared heating. The use of infrared heating in particular is important because it can be considered a ‘massless’ furnace; it is a limitation of many heat-flux DSC designs that the large thermal mass of the heating element restricts the maximum heating rate attainable. The furnace operates against a liquid nitrogen cooled heat-sink, which gives the instrument a wide performance envelope (Figure 4). In addition, Tzero® technology is employed to increase baseline performance and improve signal response. A schematic representation of the instrument is given in Figure 5 and further details of the design of the instrument are available elsewhere.5


Pharmaceutical applications of fast heating rates
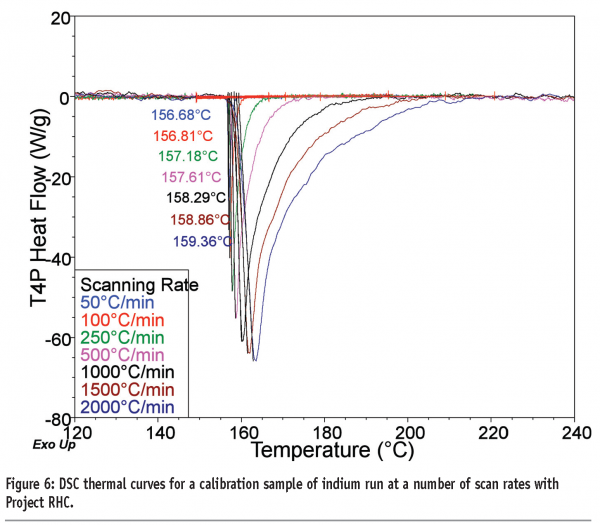
The most obvious benefit of fast heating rates is the concomitant reduction in the time required to run experiments, an important benefit in a world which demands ever-increasing throughput of samples, and a good overview is provided by Mathot et al.9 This benefit applies to all samples investigated, but is of particular advantage to those materials whose properties may change upon prolonged exposure to increased temperatures, such as amorphous products. This advantage was noted by Ye and Fiorini10 when comparing the use of TM-DSC with FS-DSC for analysis of wheat gluten protein. The second benefit is that the increased heat flow signal allows investigation of events that occur with small thermal signals (alternatively, fewer samples are required to attain the same heat flow). Figure 6 shows the thermal curves recorded for a calibration sample of indium as a function of heating rate with Project RHC, where the increased signal

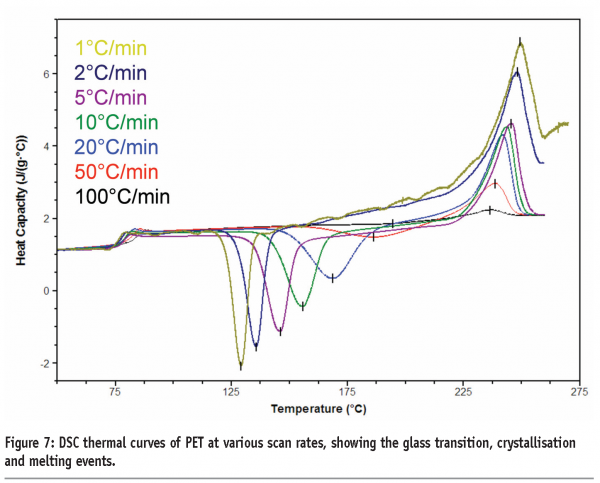
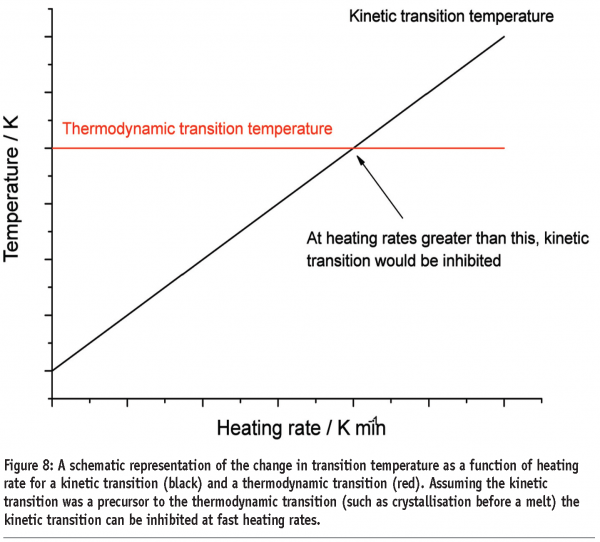
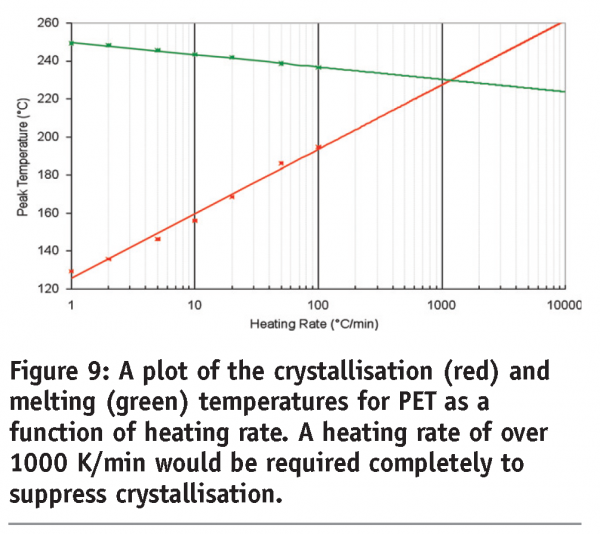
A third benefit applies to materials that undergo kinetic phase transitions (that is, a phase transition where the rate limiting step is governed by the kinetics of the event rather than the thermodynamics). In this case, the temperature at which the transition occurs will increase with increasing scan rate. The data in Figure 7 for polyethylene terephthalate (PET) serves to illustrate this point. PET exhibits a glass transition, followed by crystallisation and then melting. Both the glass transition and crystallisation are kinetic transitions, while melting is a thermodynamic event. Therefore, the glass transition temperature and crystallisation exotherms are seen to move to higher temperatures with increasing scan rates, while the melting endotherm remains at the same temperature. While initially this may seem little more than irksome, this effect has significant implications for the investigation of pharmaceutical materials and underpins the most important applications of the technique. Assuming that a kinetic transition is a precursor to a thermodynamic transition, then by shifting the temperature at which a kinetic transition occurs to a high enough temperature, the transition can effectively be inhibited. This is represented graphically in Figure 8 on page 87. At any scan rate above the intersection of the lines, the kinetic event would be inhibited because the thermodynamic event would precede it. Construction of a plot of the crystallisation and melting temperatures for PET as a function of scan rate reveals that scan rates greater than 1000 K/min are required to completely suppress crystallisation, (Figure 9). Further discussion of this type of analysis, with reference to polymers, can be found in Poel and Mathot.11



Pharmaceutically, the ability to shift or inhibit phase transitions is extremely useful. If a compound is unstable and degrades during melting, it can be difficult to assign the ‘true’ melting point, either because the signal becomes very noisy as degradation occurs or because the degradants (even in small quantities) act to lower the melting point. Other than a noisy baseline, another classic sign that a sample is degrading is that the melting endotherm moves with changing heating rates (another contribution to this effect is vant Hoff’s law of freezing point depression). Riga12 recently published a review of the use of FS-DSC to determine the stability through the melting point of a number of drugs classified in the United States Pharmacopoeia (USP) as stable.
Perhaps the most important application of this effect comes with study of polymorphs. Understanding, and more importantly controlling, the polymorphs of a new active principal is central to any drug development programme. Usually a polymorph screen will be conducted early during preformulation to discover any polymorphs of a drug. The polymorphs are then characterised, primarily with X-ray diffraction and then with DSC because it gives the melting temperature and enthalpy of fusion for each polymorph. One characteristic of polymorphism is that under a given set of environmental conditions often only one polymorph is thermodynamically stable; the other polymorphs will, over time, convert to the stable polymorph. The drug is said to exhibit monotropic polymorphism and the unstable polymorphs are metastable. During a DSC run, any metastable polymorphs will melt at a lower temperature than the stable polymorph. Following melting, the drug will recrystallise to a more stable polymorph which will subsequently melt at a higher temperature. Thus, the DSC thermal curve for what may be considered a simple sample, such as a pure metastable polymorph of a drug, may in fact turn out to be complex because the DSC data will comprise a number of exo- and endotherms corresponding to crystallisation and melting of the respective polymorphs.
If the exo- and endotherms are widely spaced and fully resolved, then analysis is not a problem. However, if the peaks start to overlap, quantitative interpretation becomes a challenge. Because FS-DSC can be used to inhibit kinetically limited phase changes such as crystallisation, it has found wide application to polymorphic characterisation of pharmaceuticals. For instance, McGregor at al13 used FS-DSC to characterise the polymorphs of carbamazepine. They noted that the enthalpy of fusion of Form III had never been determined because of its rapid conversion to Form I during heating. By heating the sample at 500 K/min, they were able to inhibit this change in form and hence determine a value for the enthalpy of fusion of Form III. Furthermore, they showed it was possible to quantify small proportions (ca. one per cent w/w) of Form III of the drug in a sample that was predominantly Form I. Further work by the same author14 resulted in determination of the enthalpies of fusion of two polymorphs of a Merck development compound, using scan rates greater than 400 K/min.
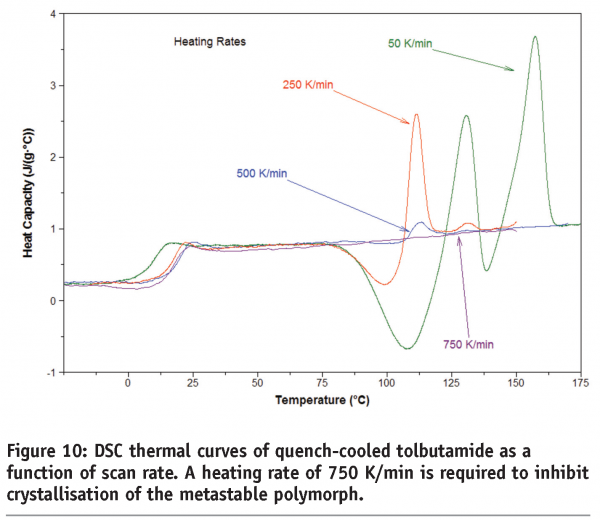

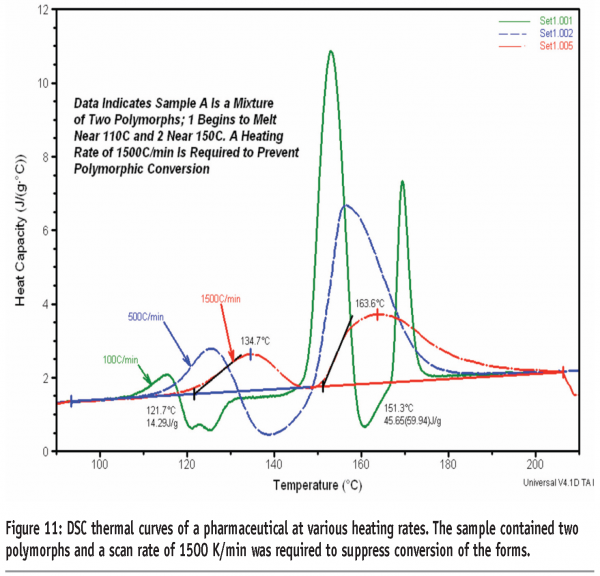
Figure 10 shows the DSC thermal curves of quench-cooled tolbutamide. Tolbutamide has four known polymorphs15 (I, II, III and IV). Form I are the stable polymorph and melts at 126-132°C. Here a glass transition is seen before crystallisation to, and melting of, Form I. It can be seen that a scan rate of 750 K/min is required in this case to inhibit crystallisation of the drug. Figure 11 shows the DSC thermal curves for a pharmaceutical sample containing two polymorphs. In this case, a minimum heating rate of 1500 K/min is required to suppress conversion of the forms.

In the same way as small proportions of a polymorph can be quantified in a heterogeneous system, fast scan rates also permit quantification of small amorphous contents. This is important because processing a pharmaceutical (such as by milling or micronising) may result in the formation of amorphous material in what was previously an entirely crystalline material. Although the per cent amorphous content formed in this way may be small (on the order of 1-2% w/w) it will be located on the surface of the material and hence may exert considerable influence over the properties of the sample. The need to quantify small amorphous contents in processed pharmaceuticals is thus pressing. The use of DSC for measuring small amorphous contents is predicated on determining the step height of the glass transition (the step seen at the glass transition temperature arises from a change in heat capacity of the sample; essentially the molecular mobility of the sample increases post the glass transition temperature). For a particular sample increasing amorphous contents result in quantitative increases in the step height of the transition.
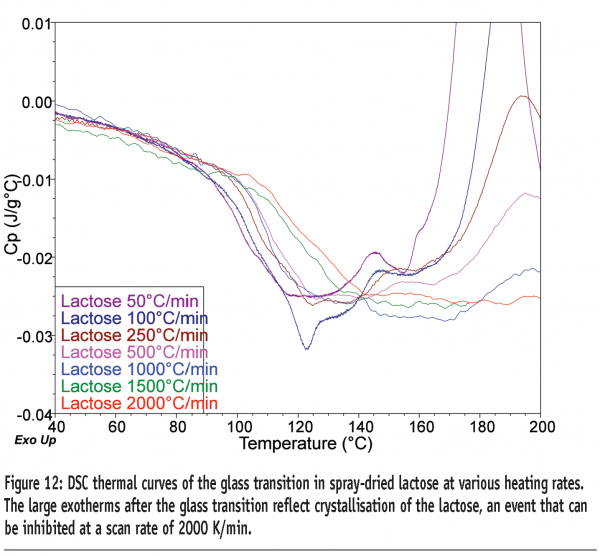

The use of FS-DSC for quantifying small amorphous contents in lactose has been reported.16 Quantitative exploration of the use of the technique showed that a limit of quantification (LOQ) of 1.89 % w/w could be obtained for amorphous content in lactose, when heating the sample at 500 K/min.17 Similar studies looking at determining amorphous contents in maltitol gave a LOQ of 0.36 % w/w.18 The data in Figure 12 show the Tg for lactose determined at various heating rates up to 2000 K/min. The sample crystallises after the glass transition at all heating rates up to 1500 K/min but is inhibited at 2000 K/min. Although not yet quantified, the increase in step-height at Tg at 2000 K/min was ca. four-fold greater than at 500 K/min. There is thus the potential to reduce the LOQ for amorphous content in lactose to around 0.5% w/w.
More novel pharmaceutical applications of fast heating rates have been reported. For instance, Buckton et al19 used FS-DSC to study wet-granulated powders (in this instance, polyvinylpyrrolidone (PVP) and lactose). They noted that it was possible to detect the glass transition of PVP in a wet-granulated blend, because of the increased sensitivity of the technique, and also that the glass-transition temperature had been plasticised (lowered), implying the binder was actually a solid dispersion of PVP and lactose, not PVP alone as would be expected. Analysis of the data suggested the ratio of PVP to lactose in the solid dispersion was 1:1.
Gramaglia et al20 used FS-DSC to measure the solubility of metronidazole in silicone elastomer. They found that by scanning at fast heating rates (400 K/min), the system was under non-equilibrium conditions, but that these gave results more consistent with solubility data determined under conventional isothermal conditions. Similar work looked at the solubility of ibuprofen in wax.21
Summary
Fast DSC heating rates offer many of benefits; shorter run times (hence faster throughput), greater sensitivity, less potential for change in the sample during heating and the ability to inhibit kinetic phase transitions. In particular, the technique is excellent for studying materials that are either amorphous or that exhibit polymorphism. Existing FS-DSC technology has been developed either on existing equipment modified to achieve fast heating rates or on solid-state devices. Project RHC is the first instrument to have been designed specifically to operate at fast heating rates but with the convenience of use of a standard DSC. Technological advancements that have facilitated this include reducing the size of the measuring cell and the implementation of ‘massless’ infrared heaters. The instrument can achieve heating rates of up to 2000 K/min, which means increased sensitivity (and thus lower limits of quantification) and the potential to expand the range of materials that can be characterised.
Acknowledgements
Dr Gaisford thanks Dr Steven Aubuchon and Mr Peter Caulfield of TA Instruments LLC for valuable discussion of the manuscript and provision of data for examples
References
- Simon SL. Temperature-modulated differential scanning calorimetry: Theory and application. Thermochim Acta, 374:55-71.
- Gaisford S, Buckton, G. High-sensitivity differential scanning calorimetry. In, Thermal analysis of pharmaceuticals, Craig DQM, Reading M (Eds), CRC Press (Boca Raton), 2007.
- Ladbury JE, Choudhry BZ (Eds). Biocalorimetry. John Wiley and Sons (Chichester), 1998.
- Gabbott P, Clarke P, Mann T, Royall P, Shergill S. A high-sensitivity, high-speed DSC technique: Measurement of amorphous lactose. Am Lab, 35:17-21, 2003.
- Danley RL, Caulfield PA, Aubuchon SR. A rapid-scanning differential scanning calorimeter. Am Lab, 40:9-11, 2008.
- Coleman NJ, Craig DQM. Modulated temperature differential scanning calorimetry: A novel approach to pharmaceutical thermal analysis. Int J Pharm, 135:13-29, 1996.
- Pijpers TFJ, Mathot VBF, Goderis B, Scherrenberg RL, van der Vegte EW. High-speed calorimetry for the study of the kinetics of (de)vitification, crystallization and melting of macromolecules
- Minakov AA, Schick C. Ultrafast thermal processing and nanocalorimetry at heating and cooling rates up to 1 MK/s. Rev Sci Inst, DOI 10.1063/1.2751411, 2007.
- Mathot VBF, Poel GV, Pijpers TFJ. Improving and speeding up the characterization of s ubstances, materials and products: Benefits and potentials of high-speed DSC. Am Lab, 38:21-25, 2006.
- Ye P, Fiorini KS. Thermal analysis of biodegradable material: From modulated temperature DSC to fast scan DSC. Am Lab, 39:25-29, 2007.
- Poel GV, Mathot VBF. High performance differential scanning calorimetry (HPer DSC): A powerful analytical tool for the study of the metastability of polymers. Thermochim Acta, 461:107-121, 2007.
- Riga AT, Golinar M, Alexander KS. Fast scan differential scanning calorimetry distinguishes melting, melting-degradation/sublimation and thermal stability of drugs. ASTM Special Publication 1466-EB, 2007.
- McGregor C, Saunders M, Buckton G. The use of high-speed differential scanning calorimetry (Hyper-DSC) to study the thermal properties of carbamazepine polymorphs. Thermochim Acta, 417:231-237, 2004.
- McGregor C, Bines E. The use of high-speed differential scanning calorimetry (Hyper-DSC) in the study of pharmaceutical polymorphs. Int J Pharm, 350:48-52, 2008.
- Simmons DL, Ranz RJ, Gyanchandani ND, Picotte P. Polymorphism in pharmaceuticals II (tolbutamide). Can J Pharm Sci, 7:121-123, 1972.
- Gabbott P, Clarke P, Mann T, Royall P, Shergill S. A high-sensitivity, high-speed DSC technique: Measurement of amorphous lactose. Am Lab, 35:17, 2003.
- Saunders M, Podluii K, Shergill S, Buckton G, Royall P. The potential of high-speed DSC (Hyper-DSC) for the detection and quantification of amorphous content in predominatly crystalline samples. Int J Pharm, 274:35-40, 2003.
- Hurtta M, Pitkanen I. Quantification of low levels of amorphous content in maltitol. Thermochim Acta, 419:19-29, 2004.
- Buckton G, Adeniyi AA, Saunders M, Ambarkhane A. Hyper-DSC studies of amorphous polyvinylpyrrolidone in a model wet granulation system. Int J Pharm, 312:61-65, 2006.
- Gramaglia D, Conway BR, Kett VK, Malcolm RK, Batchelor HK. High speed DSC (Hyper-DSC) as a tool to measure the solubility of a drug within a solid or semi-solid matrix. Int J Pharm, 301:1-5, 2005.
- Oladiran GS, Batchelor HK. Determination of ibuprofen solubility in wax: A comparison of microscopic, thermal and release rate techniques. Eur J Pharm Biopharm, 67:106-111, 2007.
† Heat is an ideal parameter to measure because it is a universal accompaniment to both chemical and physical change; very few processes occur without a change in heat so nearly any sample is open to calorimetric investigation
‡ Note that there are a number of terms in common use for DSC experiments with fast temperature scan rates, including Hyper-DSCTM (Perkin Elmer), high-speed DSC and rapid heat-cool (RHC) DSC. For simplicity, the term fast-scan will be used throughout. Note also that compared with certain branches of physics, the scan rates employed in DSC would be considered pedestrian, rather than fast!




