Real-time quantitative PCR – opportunities and pitfalls
Posted: 2 August 2008 | Stephen Bustin, Professor of Molecular Science, Institute of Cell and Molecular Science, University of London | No comments yet
The emergence of next generation sequencing technology has brought the prospect of digital analyses closer, technology that will allow not just the quantification of nucleic acids, but will result in the fine-tuning of this information with respect to tissue- and cell-specific transcription, the identification of new transcriptional units, e.g. the detection of new splice variants and their overall correlation with genomic elements. Until that time, the real-time quantitative polymerase chain reaction (qPCR) continues as the enabling technology par excellence offering an unrivalled combination of simplicity, cost-efficiency, accuracy and availability, with application in every area of life sciences and medicine1. Its sensitivity, specificity, and wide linear dynamic range makes qPCR today’s method of choice for any research and diagnostic application that aims to detect and measure nucleic acids2.
The emergence of next generation sequencing technology has brought the prospect of digital analyses closer, technology that will allow not just the quantification of nucleic acids, but will result in the fine-tuning of this information with respect to tissue- and cell-specific transcription, the identification of new transcriptional units, e.g. the detection of new splice variants and their overall correlation with genomic elements. Until that time, the real-time quantitative polymerase chain reaction (qPCR) continues as the enabling technology par excellence offering an unrivalled combination of simplicity, cost-efficiency, accuracy and availability, with application in every area of life sciences and medicine1. Its sensitivity, specificity, and wide linear dynamic range makes qPCR today’s method of choice for any research and diagnostic application that aims to detect and measure nucleic acids2.
The emergence of next generation sequencing technology has brought the prospect of digital analyses closer, technology that will allow not just the quantification of nucleic acids, but will result in the fine-tuning of this information with respect to tissue- and cell-specific transcription, the identification of new transcriptional units, e.g. the detection of new splice variants and their overall correlation with genomic elements. Until that time, the real-time quantitative polymerase chain reaction (qPCR) continues as the enabling technology par excellence offering an unrivalled combination of simplicity, cost-efficiency, accuracy and availability, with application in every area of life sciences and medicine1. Its sensitivity, specificity, and wide linear dynamic range makes qPCR today’s method of choice for any research and diagnostic application that aims to detect and measure nucleic acids2.
This process has been helped by several recent developments that have combined technology to improve qPCR assay performance and accuracy and simplify data analysis:
- the introduction of less expensive, optimised reagents that make reaction assembly simpler and more consistent;
- the development of more intuitive analysis software by both instrument manufacturers as well as by third party developers to help with assay setup and project management e.g. Prexcel (available from [email protected]) or BioGazelle’s qBase Plus (www.biogazelle.com)3;
- the introduction of advanced algorithms that allow more accurate quantification4,5,6,7,8,9,10,11.
- The extension of the technology into novel areas such as high throughput, nanoliter qPCR12. Specifically, microfluidic digital PCR is an exciting new development that extends the scope of qPCR technology13. Miniaturisation offers several potential advantages such as short assay time, low reagent usage and rapid heating/cooling rates, as well as integration of multiple processing modules to further reduce size and power consumption. Heating rates of 175ºC and cooling rates of 125ºC have been achieved14, albeit at high cost and reaction volumes as low as as 0.45 nl have been reported15. Potential problems are that as the sample volume is decreased, sample solution can easily evaporate and that amplification is increasingly prone to biochemical surface absorption problems at the chamber/channel walls due to the increasing surface-to-volume ratio. Furthermore, nucleic acid contamination in PCR chips can generate false positive results, a problem addressed by the use of a disposable PCR chip16. Clearly, there is a huge potential in clinical diagnostics for the combination of PCR microfluidic chips and qPCR, as long as the technology can accommodate a wide range of crude biological samples as analytical targets17,18. Another recent development has been termed qPCR tomography19 and combines the use of laser microdissection with qPCR analysis to obtain expression profiles from within single cells.
- The launch of less expensive, but more robust and reliable qPCR thermal cyclers, e.g. Corbett’s Rotor-Gene 6000 (www.corbettlifescience.com) or BioRad’s CFX (www.biorad.com) models. The introduction of qPCR instruments using a 384-well format (e.g. BoRad’s CFX and Roche’s Lightcycler 480 (http://www.roche.com/prod_diag_lc-480.htm) facilitates high assay throughput and, together with support for faster assay conditions, enables shorter assay times. High-density array-based formats are being developed, allowing the parallel screening of hundreds of targets at vastly reduced reagent cost. Of course, this needs to be balanced with potantial problems related to reduced assay sensitivity and accuracy of target quantification.
Clearly, from a technical point of view there are numerous developments that are strengthening qPCR technology and are permitting its application to an extremely broad range of applications. However, it is worth reflecting on these applications, and pausing to reflect on the appropriateness and biological relevance of many of the reported findings.
Targeting of DNA: qPCR
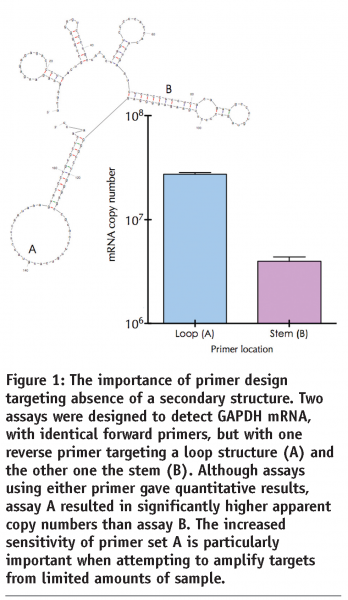
qPCR is at its most successful when used for the detection and discrimination of DNA, e.g. pathogens, translocations, mutations or SNP analyses. This is because sample handling, template preparation, assay protocols and data interpretation are all relatively straightforward. The main limitations are associated with assay design in general, and primer design in particular. It is now well established that amplification efficiency, and hence assay sensitivity, is directly associated with good primer design (see Figure 1). In practice this means designing several primer combinations for each target and testing the various permutations to obtain the optimal combination. Although this has been well documented and is easily and cheaply implemented, it is still an aspect of assay design that is all too frequently neglected. This results in many primers being used that have not been optimised, both in terms of design as well as reaction conditions. An increasingly practical alternative to assay design from scratch is the interrogation of qPCR primer and probe databases, especially if the assay targets human or mouse sequences. For example, RTPrimerDB (http://medgen.ugent.be/rtprimerdb) is a curated database of validated primers for use in real-time PCR20 and contains nearly 4,000 primer sets, 2,900 of which target human sequences, with the vast majority useful for mRNA quantification experiments; 2,400 primer sets make use of SYBR Green I, most of the others utilise TaqMan chemistry. It provides a freely accessible data retrieval system and an in silico assay evaluation pipeline for all of these qPCR assays. Specifically, assay reports contain gene information, oligonucleotide sequences, reaction conditions), publication information, users’ experimental evaluation feedback and submitter’s contact details. Additional information is provided on the alignment of primer and probe sequences on known transcript variants of a gene, along with SNP positions and peptide domain information. Importantly, the secondary structure of the amplicon sequence is predicted, as this has been reported to impact the efficiency of the PCR. If appropriate, its use helps prevent time-consuming primer design and experimental optimisation, and introduces a certain level of uniformity and standardisation among different laboratories.

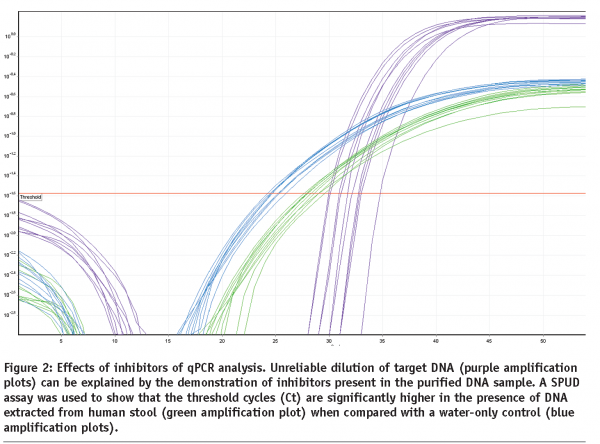
Inhibition of the PCR assay by factors co-purified during nucleic acid extraction can be an important factor contributing to poor assay performance. This is illustrated in the results of the SPUD inhibition assay21 shown in Figure 2, which shows that inhibitors remain present in DNA extracted from human stool, despite extensive purification following the instructions of the kit manufacturer. It is well worth testing every sample for the presence of inhibitors that can affect different targets, enzymes and assays in different ways.

Targeting of RNA: RT-qPCR
RT-qPCR is widely used for the detection of cellular mRNA22, micro RNAs23 as well as pathogens24. However, there are biological as well as technical limitations to its use that make its use problematic25. This is particularly so if the aim is to determine differences in expression patterns between single cells. Variability arises from the uncertainty caused by fluctuations in the amplificaton of very low numbers of target molecules as well as differences in mRNA levels between identical cells caused by the stochastic nature of transcription in mammalian cells26,27,28.
1. Biological variability
A significant contribution to variability in gene expression studies, and one that constitutes ‘baseline’ variability, arises from the fact that cellular mRNA levels are meant to be variable; after all, mRNA is constantly synthesised, localised, translated and degraded in response to extracellular signals. In vivo RNA is subject to constant degradation by complex interactions of deadenylation and decapping enzyme complexes as well as 3’-5, 5’-3’ exonucleases as well as endonucleases29. This is likely to result in significant natural variability of mRNA levels between genes expressed in different tissues and individuals. Consequently, different biopsies from the same individual as well as biopsies from different individuals will reveal a basic set of variable mRNA levels that must be taken into account when interpreting changes in mRNA levels. Each biopsy will be subject to sample-specific as ‘in vivo’ degradation over which the investigator has no control.
Another biological variable concerns gene splicing, a post-transcriptional modification in which a single gene can specify multiple proteins, allowing the synthesis of protein isoforms that are structurally and functionally distinct. This affects most human genes30 and plays an important role in human pathologies, including cancer31 and generates significant problems with the interpretation of RT-qPCR and microarray data, since presence or, indeed significant changes in mRNA levels may reflect cell, tissue- or treatment-specific adjustments between different isoforms.
The interpretation of mRNA quantification data is further complicated by the widespread differences in allelic expression among autosomal non-imprinted genes in animals32 as well as plants33. This suggests that it is not sufficient just to quantify mRNA expression, but that it is important to determine precisely which allele is being expressed. This is particularly so since allelic imbalance and allele-specific expression patterns are associated with disease risk34,35 poses further problems for. Rather than avoiding SNPs when designing primers, it may be necessary to include them as part of an overall assay design strategy so as to be able to quantitate allele-specific expression accurately25.
2. Technical variability
There are numerous reasons for technical variability although, unlike biological variability, these can be adressed by the use of appropriate standing operating procedures. The most obvious causes are inconsistent sample selection, handling, and RNA isolation. For example, a comparison of RNA levels between cancer samples must take into account the complexity and heterogeneity of tissue biopsies and may reqire the use of microdisected samples for maximum accuracy. Crucially, the accuracy of gene expression profiling is highly dependent on RNA quality, both in terms of its integrity as well as in terms of the lack of inhibitors co-purified during the extraction procedures21,36,37. The instability of RNA and its sensitivity to degradation introduced during storage or the extraction of the RNA are well-known. Whilst these comments may seem obvious, their implications have never been explored in detail. Unfortunately, not sufficient attention is paid to the analysis of RNA quality and a 2005 survey of the working practices of 100 experienced qPCR users revealed that around half did not adequately quality assess their RNA38. A recent survey of papers published in 2007/08 revealed that >60% of papers not even mentioning mRNA quality25. This area requires urgent attention, and proposals for adequate RNA integrity testing have been put forward36.
The conversion of mRNA to cDNA is probably is a highly variable step in the quantification process. RT-qPCR gene expression measurements are comparable only when the same priming strategy and reaction conditions are used in all experiments and the samples contain the same total amount of RNA39. Furthermore, reverse transcription yields vary considerably with the choice of reverse transcriptase and variation is target gene dependent40. Similarly, the mechanism of cDNA priming has a significant effect on the outcome of any quantification experiment, since gene-specific priming, random priming and oligo-dT all produce diverse results that are distinct for different mRNA targets. The choice of primer location on the target mRNA also can yield significantly different results, as mRNA adopts a tight secondary structure characterised by extensive intra-strand base pairing resulting in stem-loop structures41. If reverse transcription primers are designed to target stems, rather than loops, or if the amplicon can adopt secondary structures, the efficiency of the RT step is significantly compromised. Characteristically, this results in non-quantitative and non-reproducible results.
Proper normalisation of gene expression data between different samples generated in the same laboratory, or generated in different geographical regions using a single platform or multiple platforms is essential for obtaining accurate gene expression data42. Unfortunately, data normalisation continues to be used in an inappropriate manner, with a high proportion of papers still reporting expression patterns of target genes normalised against a single, unvalidated reference gene. This issue is equally relevant, but all too frequently unappreciated, when assessing miRNA levels43. Inappropriate experimental designs, improper analyses, subjective interpretation of RT-qPCR data, variability of microarray results depending on the choice of analysis algorithms all combine to compromise the interpretation and confident application of quantitative, mRNA-targeted data44.
Conclusions
Technological advances mean that there is an ever-increasing choice of platforms, chemistries, protocols as well as applications and targets for qPCR analysis. This is exciting and is generating a vast amount of data in, amongst others, basic research, medical, agricultural, microbiological and forensic applications. However, it is clear that a high percentage of publications utilising qPCR technology, and especially those aiming to profile cellular RNA levels, report poorly designed, executed and interpretated experiments and results41. Considerations of mRNA transcription, in vivo stability, regulation by miRNAs, tissue-specificity of splice variants, allele-specific differences in expression, the lack of concordance between most mRNAs and their specified proteins, the critical importance of post-translational modifications and questions of tissue heterogeneity all describe serious issues that are not being addressed in an adequate manner25.
Trust in the accuracy and integrity of the scientific literature is an essential prerequisite for maintaining scientific excellence and advancing knowledge. This calls for urgent action by researchers, reviewers and editors who need to agree a basic set of quality criteria and adhere to elementary procedures that result in the publication of reliable and reproducible data. Such a list must include delineating minimum quality standards for template preparation, validation and consistent use of cDNA priming methods, enzymes, protocols and, equally critically, appropriate analysis of data. Furthermore, it is entirely unacceptable that most publications do not address the critical issue of RNA quality assessment. It is equally unacceptable that data are not normalised in an appropriate manner. In addition, it is vital that data acquisition, analysis and reporting become more transparent. Consequently, it is necessary for the editors of scientific and biomedical publications to issue prescriptive checklists specifying the key information to be included when reporting experimental results. There are significant efforts underway to organise such ‘minimum information’ checklists, with the “Minimum information for biological and biomedical investigations” (MIBBI) project offering a common portal aimed at promoting gradual data integration (http://mibbi.sourceforge.net).
Another development concerns the problems associated with attempting to share qPCR data between different laboratories and users. It is important that data acquisition, analysis and reporting are transparent, thus enabling reinterpretation of data by others and helping to guarantee compliance with quality standards. Therefore, following the example of the MIAME (Minimum Information About A Microarray Experiment) guidelines adopted for microarray data, guidelines specifying the Minimal Information about qPCR experiments (MIqPCR) have been proposed. A new initiative, the “Real-time PCR Data Markup Language” (RDML) describes a structured and universal data standard for exchanging qPCR data (http://www.rdml.org/). A MIqPCR compliant RDML file should contain all measured data as well as information about the samples and targets being analysed. This data standard will contain sufficient information to understand the experimental setup, re-analyse the data and interpret the results. This is of particular importance for reliable exchange of annotated qPCR data between authors, peer reviewers, journals and readers.
Ultimately, these approaches need to be combined with more prosaic biological considerations, so that results are not a reflection of technical inadequacies and biological artifacts, but truly start describing actual differences in expression profiles between cells, tissues, individuals, disease states and treatment responses. Unfortunately, we are still far removed from this state, with a lot of intellectual and capital investment in technological development that drives research whose results can be fundamentally flawed. It will require a significant amount of courage, and a sea-change in attitude from the research community to deal with this quagmire.
References
- M. Kubista, J.M. Andrade, M. Bengtsson, A. Forootan, J. Jonak, K. Lind, R. Sindelka, R. Sjoback, B. Sjogreen, L. Strombom, A. Stahlberg, and N. Zoric, The real-time polymerase chain reaction. Mol Aspects Med 27 (2006) 95-125.
- S.A. Bustin, V. Benes, T. Nolan, and M.W. Pfaffl, Quantitative real-time RT-PCR–a perspective. J Mol Endocrinol 34 (2005) 597-601.
- J. Hellemans, G. Mortier, A. De Paepe, F. Speleman, and J. Vandesompele, qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol 8 (2007) R19.
- M.J. Burns, G.J. Nixon, C.A. Foy, and N. Harris, Standardisation of data from real-time quantitative PCR methods – evaluation of outliers and comparison of calibration curves. BMC Biotechnol 5 (2005) 31.
- O. Nordgard, J.T. Kvaloy, R.K. Farmen, and R. Heikkila, Error propagation in relative real-time reverse transcription polymerase chain reaction quantification models: the balance between accuracy and precision. Anal Biochem 356 (2006) 182-93.
- A. Batsch, A. Noetel, C. Fork, A. Urban, D. Lazic, T. Lucas, J. Pietsch, A. Lazar, E. Schomig, and D. Grundemann, Simultaneous fitting of real-time PCR data with efficiency of amplification modeled as Gaussian function of target fluorescence. BMC Bioinformatics 9 (2008) 95.
- M.V. Smith, C.R. Miller, M. Kohn, N.J. Walker, and C.J. Portier, Absolute estimation of initial concentrations of amplicon in a real-time RT-PCR process. BMC Bioinformatics 8 (2007) 409.
- A.N. Spiess, C. Feig, and C. Ritz, Highly accurate sigmoidal fitting of real-time PCR data by introducing a parameter for asymmetry. BMC Bioinformatics 9 (2008) 221.
- P. Verderio, S. Pizzamiglio, F. Gallo, and S.C. Ramsden, FCI: an R-based algorithm for evaluating uncertainty of absolute real-time PCR quantification. BMC Bioinformatics 9 (2008) 13.
- A.E. Platts, G.D. Johnson, A.K. Linnemann, and S.A. Krawetz, Real-time PCR quantification using a variable reaction efficiency model. Anal Biochem (2008).
- R.G. Rutledge, and D. Stewart, A kinetic-based sigmoidal model for the polymerase chain reaction and its application to high-capacity absolute quantitative real-time PCR. BMC Biotechnol 8 (2008) 47.
- T. Morrison, J. Hurley, J. Garcia, K. Yoder, A. Katz, D. Roberts, J. Cho, T. Kanigan, S.E. Ilyin, D. Horowitz, J.M. Dixon, and C.J. Brenan, Nanoliter high throughput quantitative PCR. Nucleic Acids Res 34 (2006) e123.
- C. Zhang, and D. Xing, Miniaturized PCR chips for nucleic acid amplification and analysis: latest advances and future trends. Nucleic Acids Res 35 (2007) 4223-37.
- P. Neuzil, C. Zhang, J. Pipper, S. Oh, and L. Zhuo, Ultra fast miniaturized real-time PCR: 40 cycles in less than six minutes. Nucleic Acids Res 34 (2006) e77.
- J.S. Marcus, W.F. Anderson, and S.R. Quake, Parallel picoliter rt-PCR assays using microfluidics. Anal Chem 78 (2006) 956-8.
- P. Neuzil, J. Pipper, and T.M. Hsieh, Disposable real-time microPCR device: lab-on-a-chip at a low cost. Mol Biosyst 2 (2006) 292-8.
- Y.K. Cho, J. Kim, Y. Lee, Y.A. Kim, K. Namkoong, H. Lim, K.W. Oh, S. Kim, J. Han, C. Park, Y.E. Pak, C.S. Ki, J.R. Choi, H.K. Myeong, and C. Ko, Clinical evaluation of micro-scale chip-based PCR system for rapid detection of hepatitis B virus. Biosens Bioelectron 21 (2006) 2161-9.
- G.V. Kaigala, R.J. Huskins, J. Preiksaitis, X.L. Pang, L.M. Pilarski, and C.J. Backhouse, Automated screening using microfluidic chip-based PCR and product detection to assess risk of BK virus-associated nephropathy in renal transplant recipients. Electrophoresis 27 (2006) 3753-63.
- R. Sindelka, J. Jonak, R. Hands, S.A. Bustin, and M. Kubista, Intracellular expression profiles measured by real-time PCR tomography in the Xenopus laevis oocyte. Nucleic Acids Res 36 (2008) 387-92.
- F. Pattyn, P. Robbrecht, A. De Paepe, F. Speleman, and J. Vandesompele, RTPrimerDB: the real-time PCR primer and probe database, major update 2006. Nucleic Acids Res 34 (2006) D684-8.
- T. Nolan, R.E. Hands, B.W. Ogunkolade, and S.A. Bustin, SPUD: a qPCR assay for the detection of inhibitors in nucleic acid preparations. Anal Biochem 351 (2006) 308-310.
- G. Pignot, I. Bieche, S. Vacher, C. Guet, A. Vieillefond, B. Debre, R. Lidereau, and D. Amsellem-Ouazana, Large-scale Real-time Reverse Transcription-PCR Approach of Angiogenic Pathways in Human Transitional Cell Carcinoma of the Bladder: Identification of VEGFA as a Major Independent Prognostic Marker. Eur Urol (2008).
- T.D. Schmittgen, E.J. Lee, J. Jiang, A. Sarkar, L. Yang, T.S. Elton, and C. Chen, Real-time PCR quantification of precursor and mature microRNA. Methods 44 (2008) 31-8.
- X. Lu, B. Holloway, R.K. Dare, J. Kuypers, S. Yagi, J.V. Williams, C.B. Hall, and D.D. Erdman, Real-time reverse transcription-PCR assay for comprehensive detection of human rhinoviruses. J Clin Microbiol 46 (2008) 533-9.
- S. Bustin, Molecular medicine, gene-expression profiling and molecular diagnostics: putting the cart before the horse. Biomarkers in Medicine 2 (2008) 201-207.
- A. Peixoto, M. Monteiro, B. Rocha, and H. Veiga-Fernandes, Quantification of multiple gene expression in individual cells. Genome Res 14 (2004) 1938-47.
- M. Bengtsson, A. Stahlberg, P. Rorsman, and M. Kubista, Gene expression profiling in single cells from the pancreatic islets of Langerhans reveals lognormal distribution of mRNA levels. Genome Res 15 (2005) 1388-92.
- A. Raj, C.S. Peskin, D. Tranchina, D.Y. Vargas, and S. Tyagi, Stochastic mRNA synthesis in mammalian cells. PLoS Biol 4 (2006) e309.
- J. Coller, and R. Parker, Eukaryotic mRNA decapping. Annu Rev Biochem 73 (2004) 861-90.
- C. Ben-Dov, B. Hartmann, J. Lundgren, and J. Valcarcel, Genome-wide analysis of alternative pre-mRNA splicing. J Biol Chem 283 (2008) 1229-33.
- C.A. Pettigrew, and M.A. Brown, Pre-mRNA splicing aberrations and cancer. Front Biosci 13 (2008) 1090-105.
- H.S. Lo, Z. Wang, Y. Hu, H.H. Yang, S. Gere, K.H. Buetow, and M.P. Lee, Allelic variation in gene expression is common in the human genome. Genome Res 13 (2003) 1855-62.
- N.M. Springer, and R.M. Stupar, Allele-specific expression patterns reveal biases and embryo-specific parent-of-origin effects in hybrid maize. Plant Cell 19 (2007) 2391-402.
- K.B. Meyer, A.T. Maia, M. O’Reilly, A.E. Teschendorff, S.F. Chin, C. Caldas, and B.A. Ponder, Allele-Specific Up-Regulation of FGFR2 Increases Susceptibility to Breast Cancer. PLoS Biol 6 (2008) e108.
- X. Chen, J. Weaver, B.A. Bove, L.A. Vanderveer, S.C. Weil, A. Miron, M.B. Daly, and A.K. Godwin, Allelic imbalance in BRCA1 and BRCA2 gene expression is associated with an increased breast cancer risk. Hum Mol Genet 17 (2008) 1336-48.
- T. Nolan, R.E. Hands, and S.A. Bustin, Quantification of mRNA using real-time RT-PCR. Nature Protocols 1 (2006) 1559-1582.
- J.M. Gallup, and M.R. Ackermann, Addressing fluorogenic real-time qPCR inhibition using the novel custom Excel file system ‘FocusField2-6GallupqPCRSet-upTool-001’ to attain consistently high fidelity qPCR reactions. Biol Proced Online 8 (2006) 87-152.
- S.A. Bustin, Real-time, fluorescence-based quantitative PCR: a snapshot of current procedures and preferences. Expert Rev Mol Diagn 5 (2005) 493-8.
- A. Stahlberg, J. Hakansson, X. Xian, H. Semb, and M. Kubista, Properties of the reverse transcription reaction in mRNA quantification. Clin Chem 50 (2004) 509-15.
- A. Stahlberg, M. Kubista, and M. Pfaffl, Comparison of reverse transcriptases in gene expression analysis. Clin Chem 50 (2004) 1678-80.
- S.A. Bustin, and T. Nolan, Pitfalls of quantitative real-time reverse-transcription polymerase chain reaction. J Biomol Tech 15 (2004) 155-66.
- J. Vandesompele, K. De Preter, F. Pattyn, B. Poppe, N. Van Roy, A. De Paepe, and F. Speleman, Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3 (2002) 0034.1-0034.11.
- H.J. Peltier, and G.J. Latham, Normalization of microRNA expression levels in quantitative RT-PCR assays: identification of suitable reference RNA targets in normal and cancerous human solid tissues. Rna 14 (2008) 844-52.
- S.A. Bustin, and R. Mueller, Real-time reverse transcription PCR (qRT-PCR) and its potential use in clinical diagnosis. Clin Sci (Lond) 109 (2005) 365-79.





