Development of cardiac cell based assays for study of cardiac disease – in with the new by automating the old
Posted: 3 December 2008 | Yuri Volkov, Paul J Spiers and Dr Anthony Davies, HCA Research Facility Manager, Trinity College Dublin | No comments yet
Approximately 45% of all deaths and 50% of all hospitalisations in the western world are a direct result of cardiovascular disease. Cardiomyocyte hypertrophy is a mechanism by which myocardial mass is increased to compensate for any elevated physical demands placed upon the heart, thus ensuring that adequate perfusion of body tissues is maintained during these periods. However, if the hypertrophic response persists, the heart enters a critical transition from compensatory to a patho-physiological de-compensatory state which eventually leads to heart failure.
Approximately 45% of all deaths and 50% of all hospitalisations in the western world are a direct result of cardiovascular disease. Cardiomyocyte hypertrophy is a mechanism by which myocardial mass is increased to compensate for any elevated physical demands placed upon the heart, thus ensuring that adequate perfusion of body tissues is maintained during these periods. However, if the hypertrophic response persists, the heart enters a critical transition from compensatory to a patho-physiological de-compensatory state which eventually leads to heart failure.
Approximately 45% of all deaths and 50% of all hospitalisations in the western world are a direct result of cardiovascular disease. Cardiomyocyte hypertrophy is a mechanism by which myocardial mass is increased to compensate for any elevated physical demands placed upon the heart, thus ensuring that adequate perfusion of body tissues is maintained during these periods. However, if the hypertrophic response persists, the heart enters a critical transition from compensatory to a patho-physiological de-compensatory state which eventually leads to heart failure.
The development of new therapeutic tools for the treatment of this condition has in many cases been hampered by the lack of biologically relevant experimental models on which new treatments can be tested.
With the advent of laboratory automation technologies, it is now possible to screen libraries comprising of hundreds of thousands of potential therapeutic agents. These new technological innovations have increased the demand for cell based experimental models that can be used in conjunction with research platforms such as High Content Screening technologies.
High Content Technologies
High content technologies represent the convergence of several mature and trusted technologies, i.e. high-resolution digital microscopy and computerised image analysis. These automated research systems are typically used in conjunction with multiplexed cell based assays labelled with target specific probes. The power of High Content technology lies in the capability of delivering highly detailed multi-parametric data and the simultaneous monitoring of a multiplicity of sub-cellular targets.
Cardiac Hypertrophy
Cardiac hypertrophy is typified by enlargement of myocardial wall mass and changes in the gross anatomical dimensions of the ventricles.
It is generally believed that the early stages of hypertrophy can occur as a compensatory response to conditions such as chronic hypertension or valvular insufficiency. Despite the fact that the hypertrophied myocardium is initially capable of maintaining adequate blood flow to body tissues and organs, if this growth persists, the enlarged heart muscle progresses to an irreversible failing or de-compensated state1. One of the major factors contributing to myocardial de-compensation is cardiomyocyte energy starvation2.
Energy starvation in hypertrophied cardiomyocytes is believed to occur as a result of a combination of factors, such as the reduction in the ratio in the surface area between capillaries and cardiomyocytes and increased myocyte diameter, see Figure 13. This in turn leads to a reduction in the exchange of respiratory gasses and nutrients between working cardiomyocytes and blood which results in reduced energy levels in these cells.
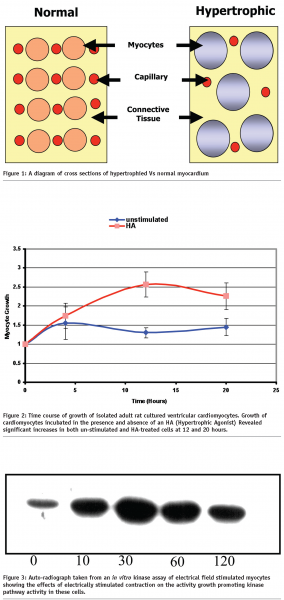

Draw backs in taking the in vivo and ex vivo approach
Over the last few decades many in vivo experimental models (i.e. whole organisms and excised hearts) have been employed to elucidate the mechanisms that underlie the hypertrophic response. Although these in vivo models have provided a useful means of studying the factors involved in this cardiac condition in the whole heart. In many cases this approach has not permitted study of cell growth specifically at the cardiomyocyte level. This is due to confounding factors that arise from the multitude of interactions between the heart and other organs and tissues within the organism.
In addition to this many of the experimental compounds used to dissect out specific biochemical processes can often be toxic or to intact organisms or produce effects in other organs and non cardiac tissues which may in turn perturb heart function. Such experimental compounds may also be difficult to administer due to poor solubility.
These issues can be avoided to some extent by using ex vivo preparations such as Langendorf perfused hearts. However, as with the in vivo (whole animal) approach, the influence of non-myocyte cell types, such as fibroblasts and endothelial cells, within the heart can be difficult to eliminate.
The need for a cell based model to study hypertrophy
It is known that cardiomyocytes constitute approximately 80% of ventricular myocardial mass and growth of these cells contributes substantially to ventricular hypertrophy in the adult heart4. Using isolated cardiomyocytes offers many advantages over tissue or organ preparations, i.e. a homogenous population of cells that can be studied in a controlled environment without interference from non-myocytes, such as fibroblasts and endothelial cells. This approach also allows for the possibility of conducting numerous experiments in parallel using cells from the same source, thus reducing experimental error.
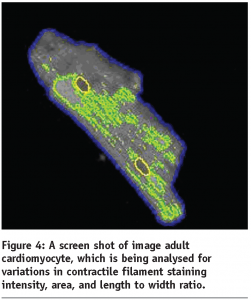
The study of the hypertrophic response in adult rat cardiomyocytes using standard biochemical techniques.
See Figures 2 and 3, and Figure 4.

Development of an assay for the study of cardiac hypertrophy using primary adult cardiomyocytes
In the early stages of this study we examined the effects of known hypertrophic stimulators on isolated adult cardiomyocytes in culture. A major finding from this work was the activation of a kinase-signalling pathway which has been implicated in cellular growth and cell survival, see Figure 3. It is also interesting to note that the blockade of this pathway by means of a selective chemical inhibitory compound reduced the cells ability to respond to hypertrophic stimuli, indicating that this pathway was involved in the modulation of this response.
Although these biochemical approaches are robust and widely used they are at best, low throughput, costly in both time and materials, and yield relatively limited information.
To gain a better understanding of cellular mechanisms that underlie this response it was clear that a high content approach might provide a faster route to achieving our experimental objectives.
In our initial studies we used HC systems to measure morphological parameters in primary adult cardiomyocytes to assess their hypertrophic status, see Figure 4. Although at first promising, it was quickly discovered that assay stability using these cells was at best, difficult to control due to short term viability and batch to batch variation.
It is for this reason that we turned our attention to the myoblastic H9C2 cell line although, H9C2 cells are not mechanically active, they do possess many key properties of primary myocytes.
Hypertrophic Response In H9C2 Cells
See Figures 5 and 6.
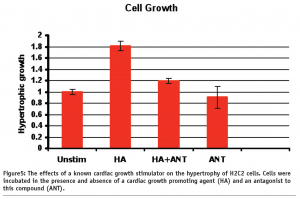


The measurement of hypertrophy in H9C2 cells
As can be observed from Figure 5, H9C2 cells treated with a hypertrophic agonist (HA) were seen to increase in size by approx 80%. Importantly, this growth response was blocked by using a specific receptor antagonist to this hypertrophic agent.
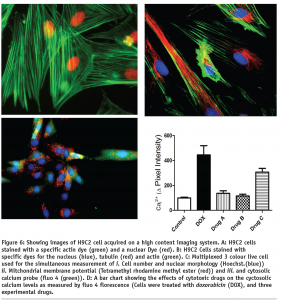
As well as this we are also examining the changes in the organisation of cytoskeletal and contractile filaments such as tubulin and actin, see Figure 6. The alignment of these fibbers has been used as an indirect marker of cardiomyocyte hypertrophy7. As can be seen from Figure 6, these cells have well defined actin and tubulin filaments, the alignment of which can be easily measured by means of high content morphology algorithms.
The transition from compensatory to de-compensatory hypertrophy
As was mentioned earlier, one of the major factors in the transition from compensated to the de-compensated hypertrophic state is energy starvation at the myocyte level, which eventually leads to cells death. Cellular energy depletion is characterised by a degradation of mitochondria membrane potential, and elevated cytosolic calcium levels. To study this process we have developed a multiplexed three colour assay, see Figure 6 on page 47, which can be used in conjunction with our high content screening platforms.
High content analysis approach makes possible the simultaneous monitoring of these key cellular parameters, allowing for the possibility of elucidating key factors in the progression of hypertrophy from the compensated to de-compensated state.
Concluding Remarks
Our studies have been focused on the development of strategies that will enable the study of a disease that is responsible for a high percentage of annual mortalities in the western word. The high content approach offers a viable means of developing new therapeutic strategies to treat this condition in a higher throughput and automatable format.
This data represents the very first stage of a larger vision for the future that involves the development of specialised cellular arrays that comprise of multiple cell types (from both model organisms such as rat and human) as well as utilising a combination of biomarkers (both functional and biochemical) that will provide a clear and inclusive picture of the sub-cellular mechanisms that are involved in the modulation of this disease process.
References
- Int J Biochem Cell Biol. 2008;40(9):1643-8. Epub 2008 Mar 18.
- J Cardiovasc Pharmacol. 1991;18 Suppl 2:S68-71
- J Mol Cell Cardiol, 20, 917-30. (1988)
- J Mol Cell Cardiol, 29, 2873-92. (1997)
- Circ. Res 69, 1476–1486 (1991)
- J. Biol. Chem. 278, 31444–31455 (2003)
- J. Clin. Invest. 103(6): 789-797 (1999).
The high content screening facility, Trinity College Dublin
The Trinity College High Content Research Facility was initially set up in 2004 as part of a larger strategic plan for Trinity Colleges’ Institute of Molecular Medicines transnational medicine centre. Since its inception, this research facility has steadily grown to a point where it now plays a central role in numerous research projects and collaborations in both the national and international scientific arenas. This research facility is now considered to be an international centre of excellence for both technical expertise and advanced training. The success of this facility has been underpinned by the efforts of our dedicated team of scientific specialists, whose primary research goal is the advancement of these technologies and associated applications. We currently service approximately 30 research projects, which range from large scale gene silencing (siRNA) screens, where the functional characteristics of each individual gene in the whole human genome is assessed by disrupting its function. Finally our facility is currently being utilised for the study of the biocompatibility of a range of advanced nano-materials which have potential therapeutic and diagnostic applications. We also have numerous projects in the areas of infection and immunity, cell signaling, lung, gut and prostate cancer, heart disease, drug discovery and applied computing.
As well as our research efforts we have recently announced the successful completion of the second year of the worlds’ first academic course in High Content Screening and Analysis, where approximately 30 postgraduate students from a range of scientific and medical disciplines receive the latest theoretical and practical training from a host of world leading experts from industry and academia.
Acknowledgments
We would like to thank the following funding bodies for their support: The Health Research Board Ireland (HRB), The Program for Research in Third-Level Institutions (PRTLI), the Trinity Foundation, and the EU-Frame Work 6 Consortium Nano interact.




