Thermal analysis and calorimetry: latest developments
Posted: 19 March 2008 | | No comments yet
Thermal analysis techniques cover all methods in which a physical property is monitored as a function of temperature or time, whilst the sample is being heated or cooled under controlled conditions. Calorimetric methods measure the energy involved in every process. The quicker new developments attain the market, such as the progression of micro or nanotechnologies, combinations of different hyphenated techniques, as well as the development of high automated or high throughput systems, the faster new horizons will open in the industrial environment. In addition, the application of sophisticated kinetic software in DSC, calorimetry and reaction calorimetry gives better safety predictions.
Table 1 summarises most thermal analysis and calorimetric methods including coupled and combined techniques, most of which have been introduced in European, US and Japan pharmacopoeias. Every new instrument leads to increased sensitivity, versatility and is constantly expanding the use of automation, therefore giving more robust and reliable results.


Manufacturers now offer all possible instruments by, integrating new pipelines which cover every field for professionals in the pharmaceutical industry, including research in life science, design of API and formulations, quality and stability monitoring in development as well as in production. Purity analysis of raw materials and packaging materials using differential scanning calorimetry (DSC) or thermocalorimetry (TG) is being introduced into production areas.
Nano or micro-DSC is routinely used in the control of proteins. In addition, special calorimeters and thermo balances are used in safety and PAT technologies.
Books and review articles dealing with the principle and instrumentation are given in references1-6.
The following sections deal with the latest developments that are having an impact on pharmaceutical manufacturing.
Physicochemical properties of API and drug products
The study of physicochemical properties present in the solid state of a drug substance, is a critical area that should be focused on in the early development stages. Different solid phases, polymorphs, solvates, hydrates or amorphous forms, of each type of salt have to be considered7. An understanding of the solid state chemistry can reduce the time to filing. Obtaining stable formulations is crucial and designing a molecular delivery system is necessary8.
Over the last decade, we have witnessed a very high increase of patent applications in regards to new crystal forms and delivery systems.
Thermal techniques play a key role since, more often then not, the crystal structure of the entities are not known. Also solvates, hydrates and anhydrous forms can only be identified by thermal analysis and microcalorimetric methods9-12,15. Today, almost every patent shows DSC and TG curves.
More active ingredients are polyphasic systems, which may appear concomitantly in the manufacturing procedure of the active ingredients. Thermal analysis techniques have demonstrated their efficiency to source thermodynamic windows for robust processes.
The combined physical analytical technique of thermogravimetric, alongside infrared analysis (TG/IR), or alternitively thermogravimetry and mass spectrometry, either technique provides an unequivocal identification of the volatile content of a pharmaceutical solid.
Variable temperature X-ray diffraction and differential scanning calorimetry methods provide the information necessary to ascertain whether the evolved gas was due to a solvent incorporated into the crystal lattice, or physically adsorbed onto the solid9,10. These techniques are not only used for the API, but also for the study of excipients and of the dosage forms5,16,17,20,21. Mechanical activation during processes, such as micronisation, induces destruction of crystallinity at the surface and unstable amorphous areas are formed. The recrystallisation of the disordered regions leads to particle growth, which has to be avoided. Conditioning after micronisation is now routinely followed by thermal analytical techniques23.
More sensitive challenging analytical tools and their impact on processes
Microcalorimetry is now an established technique complementary to DSC for the characterisation of pharmaceuticals. Larger sample volumes combined with high sensitivity, means that phenomena with very low energy levels (unmeasurable by DSC) may be studied. The output of the instrument is measured by the rate of heat change (dq/dt), as a function of time, with a higher sensitivity that is more superior to 0.1 µW.
Microcalorimetry can be applied to isolated systems that are subject to specific atmospheres or batch mode, where reactants are mixed in the calorimetre.
Solution calorimetry can be used in adiabatic or isoperibol modes in microcalorimetres, and are kept at a constant temperature. This method is used for polymorphic interpretation as well as quantitation6,17. Microcalorimetry is useful for stability and compatibility predictions, in complement to classical chromatographic evaluations24.
The most useful application nowadays though, thanks to high throughput microcalorimetre, is the routine quantitation of amorphous content, which is down to less than 0.5%17,22. The method is based on the determination of the exothermic heat obtained during the recrystallisation of amorphous material which is submitted to solvent vapours, in order to decrease the temperature of the glass transition. Since the introduction of HTS-microcalorimetry32 the development time of methods is considerably shorter.
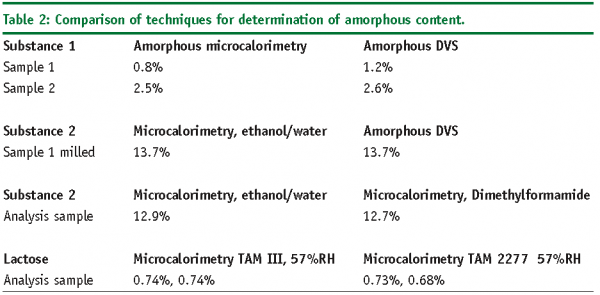
Validated methods according to ICH Q214, allows the design and quality of micronised API for inhalation purposes13,17,22. Moisture displaces the temperature of glass transition allowing amorphous material to recrystallise. For example, for a drug substance we observed approximately 8% of amorphisation. The packaging and the storage conditions revealed to be critical: if polyethylene bags (which are permeable to moisture) are used, the recrystallisation is complete after one week at 25°C/60%RH. In comparison, the same material stored in hermetic conditions remains unchanged, but if a glass bottle is used, recrystallisation occurs after two weeks at 40°C/75%RH due to the moisture contained in the air above the material. Table 2 compares some results obtained by microcalorimetry with other methods for the determination of amorphous content.

Calorimetry on a chip or micro fluid chip calorimetry, have found their first applications in biochemistry34-36.
Newly introduced automatic DVS (dynamic sorption isotherm systems)31, brings a faster understanding of complex hydration and dehydration behaviour, as well as their kinetic values with monitored relative humidity (RH %). Instruments for research have 0.1µg sensitivity and 100 mg can be used. Instrument using 1-10g samples are more adequate for large-scale production. By using solvent vapours, solvated forms can be predicted and analysed. These studies are prerequisite for the microcalorimetric analysis of amorphous content. The method has been suggested for the determination of amorphous content when the moisture is expulsed from the material at a given RH % (see Table 2).
Hyper-DSC is a very high speed method; up to 500°C/min. It allows increasing the signal of the specific heat change at the glass transition and will allow following more accurately amorphous polyphasic systems. Lyophilisates are the standard formulation in biotechnology and the amorphicity of excipients to maintain chemical stability is critical. Application of this technique for polymers and for lactose has been proved39. Since packaging materials are changing, this technique will allow better understanding and analysis of partial crystalline regions in amorphous material.
Nano-DSC and ITC is used even with HTS for the study of the stability of proteins, since the folding temperature of proteins is correlated with the biological activity27,38. They are currently used for the design and the stability studies of liposomes and emulsions. Nano-DSC (or micro-DSC) offer heating rates in range 0.001 to 1-2°C/min. or may use isothermal conditions; therefore they have wider applications such as calculations of amorphous content, stability in solutions, determinations of heat of solutions or reactions.
Automated thermogravimetric instruments are extremely sensitive and robust, combined with MS or IR, more samples can be studied resulting in more statistical relevant results being obtained. Thermogravimetry is efficiently useful in validation processes. By using derivative TG and sophisticated software, a quantitative analysis of the monohydrate of Pantoprazole in the sesquihydrate30 at the levels 6% was possible. Thermogravimetry and combined techniques are very appropriate for differentiation of hydrates. Byrn et al28 analysed samples of NF Norfloxacin and found differences. They were able to draw the schema of the inter-conversions between the anhydrous form, the dihydrate, the hemipentahydrate, the pentahydrate and the disordered state. Thermogravimetry is very efficient to optimise the drying behaviour of API and drug products.
Drug products
Interactions of components of drug products and establishment of phase diagrams are increasingly being studied. More complex delivery systems are needed for poorly soluble drugs such as solid dispersions. Stabilisation of such systems is based on the study of glass transition temperatures and their behaviour along temperature and moisture. Holt melt extrusion is a growing pharmaceutical technique29, also DSC is a very efficient tool to predict: solubility versus temperature and eutectic behaviour.
Quantitation of components of drug products can be carried out via studying the decomposition of components, or dehydration or even if no interaction occurs by the DSC peak of API. Even polymorphs can be detected in drug products by DSC down to 1% depending on the loading. DSC and TG are now state-of-the-art to follow stability of such complex delivery systems.
Sub ambient DSC is quite useful for the prediction of studies into the behaviour of polyphasic systems at a low temperature (creams, microemulsions, solvations, bound water…)
Lyophilisation of drugs and especially of proteins, is very dependent of the process design predicted by thermal analysis techniques.
The problems of interfaces between particles in solid dosage forms forced to find new ways used in material sciences. Now Microscopes are equipped with advanced environment control devices allowing heating, cooling or changing gas atmospheres40.
Micro thermal analysis (micro-TA) was introduced in 1996. Applications demonstrated that it was a reliable method for measuring transition temperatures in a highly localised manner. The instrument is capable of identifying chemical composition, distinguishing crystal habits, highlighting amorphous and crystalline areas, analysing interfaces etc. In its original form, the conventional atomic force microscopes (AFMs), were replaced by a Wollaston wire, the apex of which was etched away to reveal the platinum filament. The technique has been applied for polymers predominatly, but is adequate for formulations with polymeric excipients.
A new generation of thermal nanoprobes33 has recently been introduced with a resolution equivalent to conventional AFM imaging and sub-100 nm spatial resolution. Nano thermal analysis is a powerful adjunct to AFM and can be used in combination with other techniques such as Raman microscopy.
Design space: thermogravimetry and calorimetry as PAT tools
The increasing knowledge of physical characteristics of API and drug products needed to target challenging quality and reproducibility, should allow the design processes whereby all parameters can be precisely correlated with the desired physical property of the drug product. Once this is attained, Design Space guideline ICHQ8 drives the industry to study more accurately the processes during development.
Once the API crystallises in the designed form, mechanical processing and drug product processing have to be taken into account19,25,26.
Innovative technologies can only be successful with only the appropriate analytical techniques.
In this respect, the classical offline techniques for the analysis of the finished product (e.g. X-ray diffraction, thermal analysis and spectroscopy), are being increasingly replaced by online sensors. The industry is currently reviewing operation procedures and are looking to introduce new technologies for process monitoring.
Very well established thermal analysis and calorimetric techniques are less attractive than online spectroscopic methods. However, thermogravimetric systems are used for dryers. Pulse thermal analysis technique applied to coupled TG-FTIR, and should allow the quantification online of the FT-IR signals37.
A new calorimetre has been developed as PAT tool. Every process produces either an endothermic or exothermic change (or both). The direct measurement of change in any process would allow to control and monitor full-scale procedure. The full-scalable instrument, the ‘constant flux control calorimeter’ called Coflux R, is a new form of calorimeter, developed specifically for batch reactors that are widely used in chemical processes. The temperature of the process fluid can be modified without altering the temperature of the heat transfer surface.
This new instrument is able to detect precise endpoint reaction, prevent thermal runaways, control cooling profiles for crystallisation, measure real-time heat transfers (e.g. agitation efficiency) and also the ability to establish thermal fingerprints for each process and ensures scalability18.
Since microbial growth is accompanied by an enthalpy change, heat dissipation measured by calorimetry represents a suitable procedure to monitor metabolic activity. Both batch and heat flow calorimeters have been used to monitor processes.
Miniaturised calorimeter based on silicon integrated thermopile chips, have been proposed for this application36.
Conclusion
New developments in thermal analysis and calorimetric instrumentation reinforce the use of these techniques, which are very sensitive and offer quite a different understanding and monitoring of manufacturing processes in pharmaceutical manufacturing areas. These new techniques will efficiently increase the quality of the drug products in question, as well as the quality of the commercial chain up to customers.
References
- B. Wunderlich, Thermal Analysis, Academic Press, New York, 1990.
- P. J.Haines, Thermal Methods of Analysis, Principles, Applications and Problems, Blackie, Academic Professional, 1995.
- E.A. Turi, Thermal Characterization on Polymeric Materials; 2nd Ed. ; Academic Press, New York, 1997.
- J.L. Ford and P. Timmins, Pharmaceutical Thermal Analysis Techniques and Applications, Ellis Horwood Books in Biological Sciences, Series in Pharmaceutical Technology, Rubinstein M.H., Ed., John Wiley&Sons, 1989.
- D. Giron, Thermal Analysis of Drugs and Drug Products, in Encyclopedia of Pharmaceutical Technology, 2006, Swarbrick, J. and Boylan, J.C., eds, Marcel Dekker.
- Giron, D. (1995), Thermal Analysis and Calorimetric Methods in the characterization of Polymorphs and Solvates, Thermochim. Acta 248 pp. 1-59.
- Giron, D. (2003), Characterisation of salts of drug substances, J. Therm. Anal. Cal., 73, 441-450.
- Henck, J-O, Byrn, S.R., (2007), Designing a molecular delivery system within a preclinical timeframe, Drug Discovery Today, 12, 189-197.
- Giron, D. , (2001) Investigations of Polymorphism and Pseudo-polymorphism in Pharmaceuticals by Combined Thermoanalytical Techniques, J.Therm. Anal. Cal., 64, 37-60.
- Rodriguez C., Bugay, D.E., (1997)Characterization of pharmaceutical solvates by combined gravimetric and infrared analysis, J Pharm Sci., 86, 263-266.
- Vitez, I.M., (2004), Utilization of DSC for pharmaceutical crystal form quantitation, J. Thermal. Anal. Cal., 78, 33-45.
- Giron, D., Mutz, M., Garnier, S., (2004), Solid-state of pharmaceutical compounds:Impact of the ICH Q6 guideline on industrial development, J.Therm. Anal. Cal., 77, 709-747
- Giron, D., Piechon, P., Goldbronn, C., Pfeffer, S., (1999) Thermal Analysis, Microcalorimetry and Combined Techniques for the Study of the Polymorphic Behaviour of a Purine Derivative, J.Therm. Anal. Cal., 57,61-73.
- International harmonisation guideline Q2 on validation of analytical procedures,(1996). US29, fist supplement, 3614-3617.
- Reddy V.R., Rajmohan M.A., Shilpa R.L, Raut, D.M., Naveenkumar K., Suryanarayana, M.V., Mathad, V.T.,(2007) A novel quantification method of pantaprazole sodium monohydrate in sesquihydrate by thermogravimetric analyzer, J. Pharm. Biomed. Anal., 43,1836-1841.
- D. Giron, C. Goldbronn, Use of DSC an TG for the identification and quantification of the dosage form, J. Therm. Anal. Calorim., 48,1997,473.
- Giron, D., Monnier, S., Mutz, M., Buser, T., Stowasser, F., Schulze, K., Bellus, M, (2007), Comparison of quantitative methods for analysis of polyphasic pharmaceuticals, J. Therm. Anal. Cal., 89, 729-743.
- Hipkins, K., Advancing process solutions, Pharmaceutical Technology Europe, 2008, 1, 1-4. Ashe, R. Presentation at STK, Fribourg, 2006, Switzerland
- Brittain, H.G. and Fiese, E.F., (1999) Effects of pharmaceutical processing on drug polymorphs and solvates. In Polymorphism in Pharmaceutical Solids. H.G. Brittain ed., Marcel Dekker, New York, pp. 331-361.
- Singhal, D. and Curatolo, W., (2004), Drug polymorphism and dosage form design: a practical perspective, Advanced Drug Deliv. Rev., 56, 335-347.
- Hancock, B.C. and Zografi, G., (1997), Characteristics and significance of the amorphous state in pharmaceutical systems, J. Pharm. Sci, 86, 1-12.
- Giron, D., Remy, P., Thomas, S., Vilette, E.,(1997) J. Thermal Anal.48, 465
- Brodka-Pfeiffer, K., Hauesler, H., Grass, P., Langguth, P., Conditioning Following Powder Micronization: Influence on Particle Growth of Salbutamol Sulfate, Drug Dev. Ind. Pharm., (2003), 29, 1077-1084.
- Gaisford, S., Stability assessment of pharmaceuticals and biopharmaceuticals by isothermal calorimetry, Curr. Pharm. Biotechnol. 2005, 6, 181-189.
- Wang, SL, Lin SY,et al, Thermal behaviour and thermal decarboxylation of 10-hydroxycamptothecin in the solid state, J. Pharm. Biomed. Anal., 2007, 43, 457-463
- Salameh AK, Taylor LS, Physical Stability of crystal hydrates and their anhydrates in the presence of excipients, J.Pharm.Sci., 2006, 95, 446-461.
- N.Harn, Allan, C., Highly concentrated monoclonal antibody solutions: direct analysis of physical structure and thermal stability, J. Pharm. Sci., 2007, 96, 532-546
- W.Chongcharoen, S. R. Byrn, N., Sutanthavinu, Solid state interconversion of Norfloxacin hydrates, J. Pharm. Sci., 2008, 97, 473-487
- Repka, M. et al, Pharmaceutical Applications of Hot-Melt Extrusion: Part II, Drug Devel. And Industrial Pharmacy, 2007, 33, 1043-1057
- Reddy V.R., Rajmohan M.A., Shilpa R.L, Raut, D.M., Naveenkumar K., Suryanarayana, M.V., Mathad, V.T.,(2007) A novel quantification method of pantaprazole sodium monohydrate in sesquihydrate by thermogravimetric analyzer, J. Pharm. Biomed. Anal., 43, 1836-1841.
- Automatic multi-vapor gravimetric sorption analyzer for advanced research applications. Surface Measurement Systems Ltd, UK.
- Mutz, M., Motreff, A., Monnier, M., Buser, T., Schwab, P., Giron, D., Use of highthroughput microcalorimeter for faster determination of amorphous content, STK annual meeting, June 2006, Fribourg, Switzerland.
- Ye, J., Reading, M., Gotzen, N., van Assche, G.,(2007), Scanning Thermal Probe Microscopy: Nano Thermal Analysis with Raman Microscopy,, Microscopy and Analysis, 21, S5-S8.
- Lerchner, J., Wolf, A., Hüttl, R., Wolf G., (2004), Direct monitoring of biochemical processes using micro-structured heat power detectors, Chem. Eng. J., 101, 187-194.
- Lerchner, J., Wolf, A., Wolf G., Baier, V., Kessler, E., Nietsch, M., Krügel, M., (2006), A new micro-fluid chip calorimeter for biochemical applications, Thermochimica Acta, 445, 144-150.
- Higuera-Guisset, Rodriguez-Viejo, Chacon, M., Munoz, F.J., Vigues, N., Mas, J., Calorimery of microbial growth using a thermopile based microreactor, (2005) Thermochimica Acta, 427, 187-191.
- Eigenmann, F., Maciejewski, Baiker, A., Quantitative calibration of spectroscopic signals in combined TG-FT-IR systems, (2006), Thermochimia Acta, 440, 81-92.
- Differential Scanning Calorimetry (DSC) and Protein Stability, see applications literature in www.microcal.com
- Saunders, M., Podluii, K., Shergill, S., Buckton, G., Royall, P., The potential of high speed DSC (Hyper-DSC) for the detection and quantification of small amounts of amorphous content in predominantly crystalline samples, (2004), Int. J. Pharm., 274, 35-40.
- Advanced Environmental Control for Microscopy, Surface Measurement Systems Ltd, UK.
Danielle Giron
Chemical Research & Development, Novartis Pharma AG, Basel, Switzerland
Dr. Danielle Giron studied engineering at the School of Industrial Chemistry and Physics in Lyon, parallel to this she earned a M.S. in Chemistry and her Ph.D. ‘docteur es sciences’at the University of Lyon for Applied Chemistry. She joined Sandoz in 1971, to establish the Laboratory of Thermal Analysis and Polymorphism. Danielle has fulfilled various positions in both analytical and Research & Development at Novartis gaining a broad experience of the entire development processes, from research to production for drug substances and products. She is currently teaching at several Universities, and has had approximately 60 publications in scientific journals or chapters in scientific books. Danielle also speaks regularly at conferences all over Europe and the U.S. She is President of the Swiss Society for Thermal Analysis and Calorimetry (STK). In 1992, Danielle received the STK Award and the Novartis Leading Scientist Award 2000. She is presently Group Head in Chemical and Analytical Research & Development and is also a docent at the University of Basel.




