New model could be used to predict shelf life of drugs
Posted: 23 July 2019 | Victoria Rees (European Pharmaceutical Review) | No comments yet
Researchers have developed a model that describes how antibody solutions separate into different phases and could be used to anticipate the stability and shelf-life of drugs.

A new mathematical model, which describes how highly concentrated antibody solutions separate into different phases, in a similar way to water and oil, has been developed. This separation can reduce the stability and shelf life of some drugs that use monoclonal antibodies.
if these parameters can help us predict stability and shelf life, we may be able to select better drug candidates
The team from Penn State University, US and MedImmune, LLC (now AstraZeneca) investigated thermodynamics and kinetics using an innovative method that enables the rapid study of multiple samples at once.
“Phase separation is one of the pathways that makes these drugs unstable and unsuitable for use. The classical method to understand this process involves manipulating the temperature of one sample over time. We used a temperature gradient microfluidics platform to quickly look at many temperatures simultaneously,” said Bradley Rogers, first author of the paper.
The team used an innovative device that creates a range of temperatures across a temperature gradient and a technique called dark-field imaging to measure how quickly separation occurs. They then calculated a variety of parameters to better understand the thermodynamics and kinetics of the system, including the temperatures at which transitions occur and the energy required to go from one phase to the next.
“We observed that the rate that a solution separates into two phases has a strange dependence on temperature,” said Rogers. “This relationship is much more complicated for concentrated antibody solutions than it is for other systems. We spent a long time trying to make sense of the data, but we eventually developed a model that explains what we are seeing.”
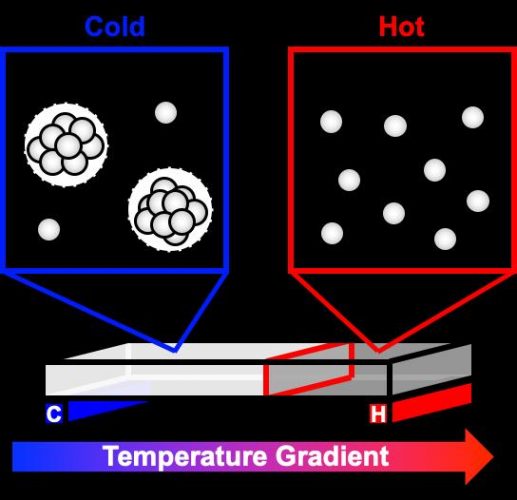

A new study used a temperature gradient to observe how highly concentrated antibody solutions, like those common in some drugs, separate into phases, like an oil and water solution. At colder temperatures, cloudy droplets begin to form in the once clear solution. As the droplets grow and settle to the bottom of the container, the solution separates into two distinct phases. This phase separation can affect a drug’s shelf life and stability [credit: Cremer Lab, Penn State]
The model describes the method used by antibody molecules to clump together as the temperature decreases, forming droplets that grow as additional molecules join. This reversible process happens more rapidly with decreasing temperature as the solution gets more saturated with free antibody molecules. When the solution continues to cool, droplets stick to other droplets and settle at the bottom. At even colder temperatures, the solution forms a gel.
“In a single experiment, we can visualise the homogenous clear solution, the cloudy solution as droplets begin to form, the phase-separated liquid and the gel,” said Paul Cremer, senior author of the paper. “Previous research described these different states and our model describes the mathematics and temperature-dependent kinetics behind what we believe is happening.”
“If these parameters can help us predict stability and shelf life, we may be able to select better drug candidates,” said Rogers. “We may also be able to determine the ideal solution properties for a promising drug candidate to keep it stable.” Next, the research team plans to investigate if their model can explain phase separation in other systems. They also plan to test whether parameters gathered from this type of experiment can forecast stability and shelf life of therapeutics.
The findings were published in Proceedings of the National Academy of Sciences.
Related topics
Antibodies, Drug Development, Formulation, Imaging, Research & Development (R&D)
Related organisations
AstraZeneca, MedImmune, Penn State University, Proceedings of the National Academy of Sciences




![Roche logo sign lit up [Credit: testing/Shutterstock.com].](https://www.europeanpharmaceuticalreview.com/wp-content/uploads/Roche-3-400x187.jpg)




