Unlocking the potential of new technologies, such as Lab-on-a-chip, to cut manufacturing costs and boost product development
Posted: 14 September 2018 | Dr Nasr Asfahani | No comments yet
The investigation and development of new drugs is a time-consuming and rigorous process with many challenges. Every step and each new method is developed with the intention of bringing effective medicines to patients in the shortest possible time, while ensuring the highest possible level of safety. Good product design, as well as good manufacturing practice (GMP), can lead to more efficient development. Aside from the mass manufacturing process, the integrity of the bioprocess itself may also limit product quality in the final stage.
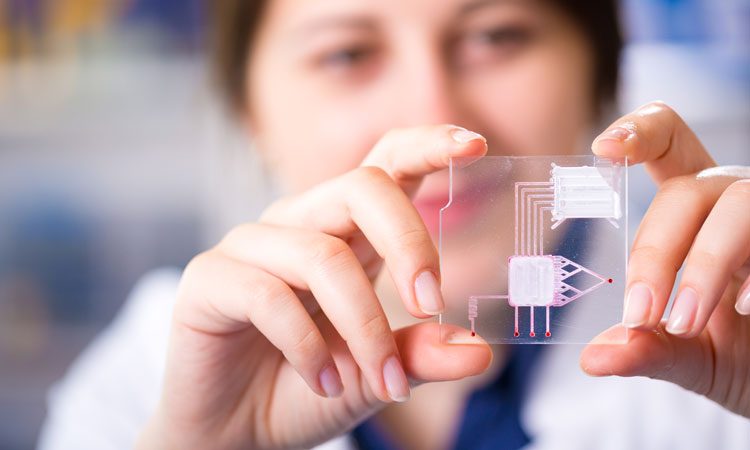

New technologies such as Lab-on-a-chip (LOC) have economic advantages of lowering the costs of manufacturing processes and improving the efficiency and accuracy of product development. Lab-on-a-chip is currently one of the most powerful technologies employed, with huge potential to impact healthcare, medicine and biopharma. After much development over the past quarter of a century – and with commercialisation starting to take off1 – the ability to shrink sample quantities to micron levels has the potential to speed up the study of many drugs designs and biopharma products. For example, LOC enables automated liquid injections using microchannels down to 50μm in width to transfer picolitre amounts of liquid in milliseconds.
LOC can also automate the process by directing the right amount of liquid at the right time using micropumping and microvalves. The flexibility of the design of these devices allows the transfer of benchtop lab bioprocesses (such as filtrations and membrane-based filter technologies) to LOC, especially in the cell separation field. For example, instead of using lengthy laboratory-based techniques and image processes to roughly estimate the number of cells in a sample, LOC – by means of a simple manufacture-able microstructure in the form of a polymer with enough hydrodynamic and surface properties – can sort and / or accurately count a large number of cells in a shorter amount of time.2 From a biopharmaceutical product development point of view, if such a device can be commercialised to accurately sort and / or filter cells, it could revolutionise health and diagnostic fields – such as perfusion bioreactors for continuous manufacturing of monoclonal antibodies3 and / or cell population heterogeneity and morphology analysis.4 When looking more in depth to design and development in the biopharmaceutical industry, there are several main aspects to be considered: these include design criteria, order of importance, quality, concentration, productivity, yield / conversion and type of bioreactor, as well as the material to be used.5 All these aspects can change depending on the biological processes being developed, such as anchorage dependence or suspension adapted and temperature gradients.
Looking at which tools have been used in the past decade to speed the process of technology transfer into manufacturing, we perceive two main directions: Process Analytical Technology (PAT) and the development of platform technologies. The well-established quality axiom that “quality cannot be tested into products but should be built-in or should be by design” is now being applied with PAT, as the operative mechanism of assuring quality, reducing failures and mitigating deviation during manufacturing.5 Additionally, thorough awareness of the manufacturing process and development path that led to it allows for flexible management of change. Computational modelling appears to enable more accurate analysis in the field, which can help the product advance more quickly. These trending analysis tools are still not sufficiently well adapted to real samples or clinical conditions of testing, but nevertheless PAT is likely to add value to process understanding and reduce the risks associated with the impact of seemingly small process changes, such as the change of a vendor for a process ingredient.5


It is important to emphasise the need to speed up the development of LOC technology with more industrialised methods and regulations on mass manufacturing standards. As with every new technology, there are unknown challenges in the design process, as well as the manufacturing line, for a prototype to reach approval level. Traditionally, many industries follow the approach of “do it right the first time”. However, being a new technology, LOC follows a more scientific approach, which can be challenging due to the restrictions of the more technical industrial approach. Traditional methodological routes are still used in the field of manufacturing, which block or slow down the process (injection moulding, optimisation of process and restriction of materials that adapted with this method). These routes are often not the best way to efficiently optimise the product development stage that aims to reach the market. In the absence of a good manufacturing practice being established, the accurate design cannot be proved biologically; hence, the manufacturing process will likely take a long time and, in many cases, will fail.
Another important aspect is the development of a production process, as well as the building of a production plant, which must be available for clinical phases. The diagnosis and biopharmaceutical industry must invest heavily in process engineering and plant construction, particularly in the fields of manufacturing, to provide valuable devices or prototypes on time for clinical studies. An example of this is organ-on-a-chip (devices that can model the effects of a drug directly into one part of body). However, it is unknown whether this product will ever reach the market and some devices will not easily lead to a return of R&D costs – especially in start-ups, as they have to invest a lot on developing technology to adapt to current standards. Ideally, one would start investing into a commercial plant when the phase of clinical study data becomes available. However, phase III clinical material must come from the commercial plant and the production process must meet regulatory requirements (for example, for the European Medicines Agency (EMA) and GMP standards).6 Therefore, many companies must develop new concepts that start process development and engineering right after the first results of clinical phase I studies.
In general, the time required for clinical studies can be long and, in many cases, design will fail by not providing the required accuracy. At the same time, the regulatory requirements are growing, so the clinical testing time may even increase. The time needed for phase III clinical development depends on the type of device designed for the developed drug and the experimental readout.
Overall, development of the LOC field still lacks financial investment due to the lack of expertise and / or manufacturing technology that could help to better produce the desired product. Often researchers working with LOC fail to recognise the technical challenges that exist when engineering a product and the activity of many companies is based on pure research, with a focus on patients, being determined to create meaningful therapies but lacking the product development component. In terms of the regulatory approval level, LOC devices are still at an early stage and the majority does not even reach commercialisation level, as most of them were not designed with this aim, but rather for scientific research (such as PhD projects). At universities, large amounts of money have been spent in LOC. Most of the projects, however, do not have an industrial aim, as this can be time consuming and development of the devices tends to finish once the project reaches the solution stage. Therefore, without any direction towards high volume manufacturing, it would be hard to standardise and tune these projects within market and industry.
In conclusion, biopharma needs to update some traditional methods. Too often nowadays, industries such as high-tech start-ups fail at the stage of regulatory approval and product marketing. Merged technologies such as LOC, which are still not fully introduced into industry and adapted within current standards and regulations, also need to be established. If biopharma requires a boost for faster development, one solution would be significant investment into the advance of design and manufacturing to allow new technologies to deliver new products more quickly.
REFERENCES
- Lab-on-a-chip workshop activities for secondary school students. Esfahani MM, et al. 2016.
- Design of a microfluidic platform for monoclonal antibody extraction using an aqueous two-phase system, Silva, et al. 2012.
- Deconstructing stem cell population heterogeneity: Single-cell analysis and modeling approaches, Wu J, Tzanakakis ES. Biotechnol Adv. 2013 Nov 15.
- New Challenges for Biopharmaceutical Process Development. Curling J.
- https://www.pwc.com/gx/en/pharma-life-sciences/pdf/pharma-2020-supplying-the-future.pdf
- Phase-Appropriate GMP. Schmitt S. Pharmaceutical Technology, Volume 40, Issue 3, 2016.
- Sweeney N, Goss T. The Value of Innovation in Oncology: Recognizing Emerging Benefits Over Time. Boston Healthcare Associates, May 2015.
- PhRMA, Biopharmaceutical Industry Profile 2017, (Washington, DC: PhRMA, April 2015).
- Jacoby R, et al. Advanced Biopharmaceutical Manufacturing: An Evolution Underway. Deloitte, May 2015.
- Lechleiter J. President’s Inaugural Address: International Federation of Pharmaceutical Manufacturers & Associations (IFPMA) 26th IFPMA Assembly. http://www.lilly.com/news/speeches/Pages/121031.aspx.
- Meanwell C. The Medicines Company website homepage, http://www.themedicinescompany.com/.
- Mukherjee S. The Emperor of All Maladies: A Biography of Cancer. 2010.
- President’s Council of Advisors on Science and Technology, Report to the President on Ensuring American Leadership in Advanced Manufacturing, Pharmaceutical Research and Manufacturers of America (PhRMA) Report, “Researching Cancer Medicines: Setbacks and Stepping Stones” http://www.phrma.org/sites/default/files/pdf/2014-cancer-setbacks-report.pdf.
BIOGRAPHY
DR NASR ESFAHANI is a designer in microfluidic and lab-on-a-chip devices with a strong interest in design for manufacturing and mass production. Nasr is passionate about creation and innovation in science and engineering, which have led him to be involved in the development of high-tech revolutionary products over the past 10 years. In 2014, he received his PhD in Engineering from the University of Hull, after being awarded a doctoral scholarship to investigate mass manufacturing strategies for lab-on-a-chip devices. He has worked in the Institute of Innovation and Product Design at the International Manufacturing Centre of the University of Warwick in collaboration with Silson Ltd. He then joined a team of scientists as a PostDoc, to develop microfluidic platforms for dose-on-demand positron emission tomography (PET) tracers at University of Hull. Currently, he is working as senior design engineer on microfluidic devices at Orphidia Ltd.
The rest of this content is restricted - login or subscribe free to access


Why subscribe? Join our growing community of thousands of industry professionals and gain access to:
- bi-monthly issues in print and/or digital format
- case studies, whitepapers, webinars and industry-leading content
- breaking news and features
- our extensive online archive of thousands of articles and years of past issues
- ...And it's all free!
Click here to Subscribe today Login here









