Pharma Digitalisation: Challenges and opportunities in transforming the pharma industry
Posted: 30 May 2017 | | 3 comments
Pharma Digitalisation Executive Consultant, Pasi Kemppainen, and Orion’s CDO Sammeli Liikkanen talk technologies, challenges and opportunities with the digitalisation of pharma…


Digitalisation is fundamentally changing the healthcare industry. The Pharma industry, as a core part of healthcare, is no exception to this. New technologies and innovations are already enabling pharma companies to improve medicine development and patient care.
At the same time, healthcare payers and other customers of pharma companies are demanding more and better data on the medication efficacy and improved patient quality of life. These demands cannot be fulfilled by purely traditional means.
Biomedicines, orphan drugs and advanced medical devices
Now that the era of conventional small molecule blockbuster medicines is about to end, the big pharma focus is shifting towards high value but lower volume products such as biomedicines, orphan drugs and advanced medical devices. The increasing price pressure caused by the tightening market price regulations and patent expirations is forcing pharma companies to challenge their current product and market strategies in order to survive.
Also, the global and heterogeneous regulation is increasing and bringing more complexity in compliance in product supply infrastructures and operations. For example, the US FDA’s vision is quality manufacturing without the extensive regulatory oversight which will create completely new kinds of requirements in the product supply capabilities and systems.
One might say that Pharma business is undergoing a perfect storm: a concurrent transformation on multiple, unrelated areas changing the whole product lifecycle from early drug development to manufacturing and patient care. This transformation will not only challenge the incumbents but also create opportunities for new entrants outside of pharma business whose innovation and business clock speed are on a completely different level.
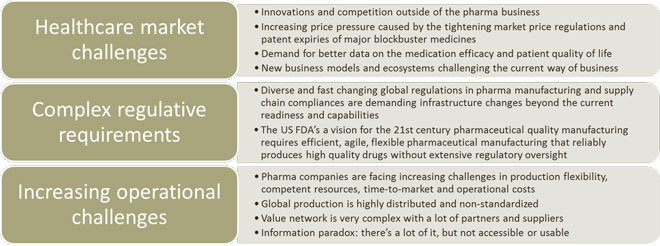

Figure 1. Pharma digitalisation transformation drivers
It is obvious that these market challenges and requirements can’t be met with the current products, business models and operations. Therefore the pharma business transformation is inevitable which will lead to the digitalisation of the pharma products, business models, operations and ultimately – the patient care. Enter the era of Pharma Digitalisation.
‘Beyond the Pill’ brings unprecedented opportunities


The most profound part of the business transformation, called Beyond the Pill, is already cutting to their core businesses in clinical development and patient care. Pharma companies are facing immense challenges on improving patient outcomes at the same time with the intensifying competition and pricing pressures.
The next generation of pharma products already available vary from patient specific biomedicines to medical devices with the capability to provide patients real-time information about their condition, and collect patient data for care analytics to improve the treatment.
These new technologies not only can help companies to address several major care challenges—such as compliance and chronic disease management—but also can help them to create hundreds of billions of dollars in value.
This has already led the pharma manufacturers capitalising the Beyond the Pill business transformation. For example, pharma giants like Novartis, GSK and Novo Nordisk are already investing in partnerships and new business models with technology companies such as Google, IBM and Qualcomm. Not to mention traditional device manufacturers like Apple, Samsung and Nokia who are already researching beyond the wellness products and looking to the patient care market.
All this will substantially improve the personal medication development and care processes where the patient and care data is the new source of innovation and competitiveness.
The new Pharma reality challenges future competitiveness
A study by NNE, a leading pharmaceutical engineering company, investigated global pharma companies’ perspectives of the future successful pharma manufacturing sites. The study identified changing expectations of success moving from site stability to site agility with three key requirements:
- ‘Flexible production’, a site’s ability to accommodate changes in production demands,
- ‘Integrated quality’, balanced and integrated quality systems; and
- ‘Entering new domains’, having the ability to quickly absorb knowledge to implement new practices.
This means that pharma companies already clearly perceive that their future competitiveness is at stake due to external and internal drivers.
In addition, the pharma manufacturing infrastructure implementations are already highly complex with an increasing number of connected equipment, and internal and external system integrations. The pharma ecosystem is highly networked with the pharma industry specific requirements eg, for the collaborative R&D data management and supply chain regulatory compliance reporting.
These fundamental pharma ecosystem, business model and technology changes are the factors leading to a new manufacturing and product supply industry transformation called the New Pharma Reality.


Figure 2. The New Pharma Reality external and internal drivers challenging the pharma industry. (Copyright of NNE)
The Pharma digitalisation ecosystems
The New Pharma Reality and Beyond the Pill business transformations will affect the whole product life cycle, from R&D to product supply and patient care, and eventually it will bring patients and their quality of life to the center of the pharma business. This industry transformation, Pharma Digitalisation, consists of two major concurrent and complementing digital ecosystems: Pharma IoT (Internet of Things) and Pharma Industrial Internet (or Industry 4.0 more generally).
The best way to conceptualise the Pharma Digitalisation is to describe it from the product lifecycle and product supply network point of view: how getting the medicines from development to patients will improve and change. This also helps with understanding the differences between Pharma IoT and Pharma Industrial Internet ecosystems.
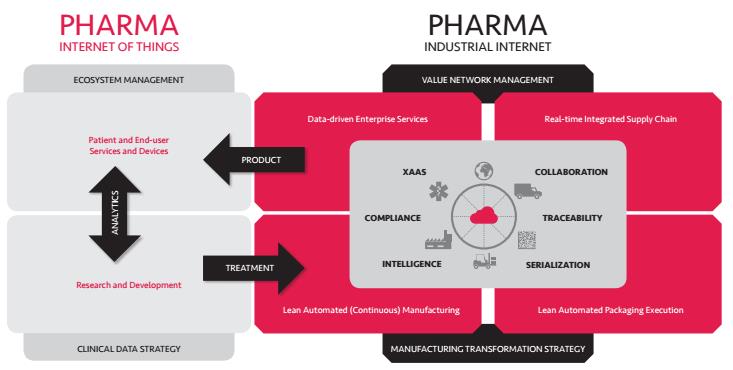

Figure 3. Pharma digitalisation ecosystems: Pharma IoT and Pharma Industrial Internet
Pharma IoT
Pharma companies have long ago realised that selling traditional medicines will not guarantee sufficient growth nor even sustain the competitiveness. This fundamental change moving Beyond the Pill typically arises from one or two realisations:
1) medicines alone are often not enough for patients to achieve optimal clinical outcomes, and
2) as the current product pipelines dry up, Beyond the Pill businesses can be valuable new sources of revenues.


This has created the interest for utilising the new technologies and business processes in development and patient care leading to Pharma Internet of Things or Pharma IoT.
Pharma IoT conceptualises the digitalisation of medical products and related care processes with smart connected medical devices and IT services (cloud, mobile, apps, etc.) in drug development, clinical trials and patient care. The outcome of the Pharma IoT in the development and clinical trials will be using the combination of advanced technologies and services for creating totally new kinds of disease treatment possibilities.
The fundamental difference between patient care Pharma IoT with other consumer IoT solutions is that pharma solutions require regulatory compliance. This distinguishes the Pharma IoT solutions from general wellness and fitness applications where the software and hardware are basically free from the regulatory screening and hence can’t be prescribed as a part of the clinical patient care.
Regulatory approval
In fact, the US FDA is already in the process of creating a separate digital health unit to validate the software for the clinical purposes. The unit will oversee patient care software using artificial intelligence, advanced analytics, cloud, wireless medical devices, telemedicine, and cybersecurity.
Pharma IoT will enable the patients and healthcare professionals to use medicines with advanced sensor hardware and personalised care services and processes. It is clear that these new capabilities will facilitate new business opportunities for the pharma companies with the existing products as well as start-ups seeking for new disruptive platforms and business models.
Wearable technology
Good examples of the Pharma IoT solutions are the connected sensor wearables for Parkinson’s disease and multiple sclerosis patients with the medication management improving the patient outcomes and the quality of life. In addition, the existing medical device products such as inhalers and insulin pump systems can be improved with sensor and connectivity technologies to collect data for further care analytics and personalised therapy.
Machine learning and artificial intelligence (AI) will naturally find their way in the patient care as well. At some point of time there will be virtual clinical care service providers helping the patients to use the collected sensor data along with the artificial intelligence for improving the care outcome for example with Parkinson’s or diabetes patients. Most probably there will be even ‘just an app’ for that. No doubt AI will also change the role and job descriptions of health care professionals in their everyday work.
At the same time, all this will enable substantially improve the personal medication and care processes where the patient care data is the new source of innovation and competitiveness for the pharma companies as the real world evidence.
And to make the transformation even more challenging, pharma companies need to take into account the forthcoming EU data protection and privacy legislation, which will give control of their care data to patients. For example, patients will be allowed to transfer their care and health data across the service providers leading to the emergence of totally new kinds of service platforms and business models.
Pharma industrial internet

Pharma Industrial Internet, like Industry 4.0, conceptualises the digitalisation transformation of the pharma product supply infrastructures from manufacturing the medicines to dispensing the medicines to patients. In other words, the industrial internet infrastructures manufacture and supply some or all medicine/sensor/device/apps/services/patient care processes capabilities to the market whereas IoT is the ecosystem and market for utilising, analysing and monetising the use of the medical products and ultimately, patient care data.
The key infrastructures and capabilities in Pharma Industrial Internet are (Figure 3):
(1) manufacturing intelligence (continuous and automated ‘lights-out’ manufacturing);
(2) software controlled packaging execution (serialisation and automation/robotisation);
(3) integrated supply chain (traceability and collaboration); and
(4) enterprise back-end IT cloud based services (XaaS) utilising the data from the product supply (analytics, life-cycle management and regulatory compliance reporting).
In addition to the manufacturing and service infrastructures, Pharma Industrial Internet will enable drastic changes in business models and global manufacturing operations. With the high level of automation and real-time global ecosystem connectivity, the medicine manufacturing and packaging can become globally transparent and distributed in more controlled fashion given the regulatory constraints and requirements.
The third-party manufacturing and global product supply could be then managed centrally in real-time by the marketing authorisation holder, and yet the products can be traced and verified on a sales unit level throughout the supply network even up to the point of dispense and patient. In the longer term, this can lead to a platform economy where the ecosystem and innovation management will be the source of the competitive advantage rather than owning the manufacturing and product supply assets.
Pharma digitalisation facilitates the healthcare transformation


Ultimately, Pharma digitalisation will help the pharma business become more patient centric. Patients will gain more possibilities for better and cost-effective care outcomes.
For example, patients with diagnosed or potential heart conditions could have in the future small embedded wireless sensors to detect cerebral infarctions at an early stage, and also overburdening and prolonged stress. The sensor data can be used for motivating patients to improve their lifestyles and habits leading to a better quality of life. This way the medication could be even avoided, and potentially the very expensive intensive care in a case of infarction. Not to mention the collateral costs and risks associated in the patient recovery process. The best and cheapest protection is indeed the disease prevention where the digitalisation will be an essential enabler for the patient centricity.
In addition, with digital solutions patients will be better informed and will be participating in their own care with the practitioners to ensure that care decisions respect patients’ wants, needs and preferences. Digital collaborative services can bring transparency and facilitate patient centered activities in the care between the stakeholders while maintaining shared up-to-date data on the patient health and quality of life. This will lead to a better care results as well as patient engagement in the care process.
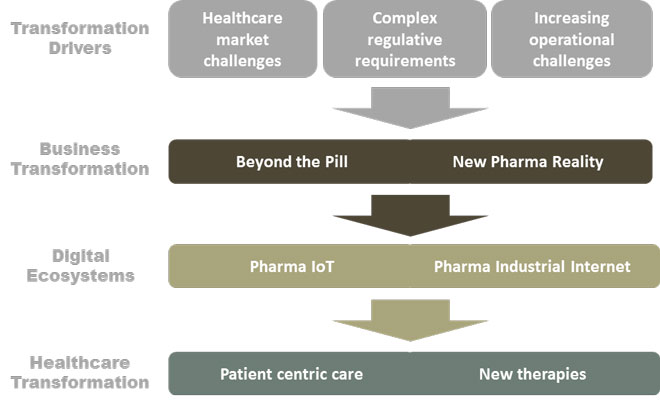

Figure 4. Pharma digitalisation facilitates the healthcare transformation
Pharma Digitalisation is transformation, not disruption


The Pharma industry is facing the biggest transformation since the rise of the modern medicines in the nineteenth century and the introduction of the manufacturing automation in 1980s. The transformation is already driven by the increasing global regulation, intensifying competition, demanding payer requirements and increasingly complicated product supply ecosystems.
At the same, completely new business opportunities are opening with new novel technologies and partnerships with high-tech companies. The future defining challenge will be how the inevitable very diverse and complex business requirements can be implemented with the current manufacturing, product supply networks and – most significantly – business models and products.
The good news is that no Uber or AirBnB like industry disruption is required to seize the digitalisation opportunities. Pharma companies simply need to start transforming into digital businesses to be competitive in the future.
After all, we should already know by now from the human history that it is not the strongest that survive, nor the most intelligent, but the ones most responsive to change.
Disclaimer: The views presented are authors’ personal opinions and do not reflect those of employer or customers.
Related topics
Big Pharma, Biologics, Drug Development, Drug Manufacturing, Patents










Thanks for the detail information about pharma digitalization challenges. New technologies and innovations are already enabling pharma companies to improve medicine development and patient care.
Digitalisation can fundamentally change the healthcare industry. New technologies and innovations are already enabling pharma companies to improve medicine development and patient care.
Think Web 2.0, mobile interfaces, e-Portals, VR, AR, AI, blockchain, realtime health monitoring, big data, customer centricity, closed loop marketing, immersive engagement, data visualisation and analytics – all the current buzzwords in digital transformation can be mentioned as examples and all are represented in and transforming the pharma industry.
pharmaceutical companies have turned to botanical drugs for alternative medicines to treat diseases with unmet medical needs. This is really knowledgeable blog and easily understandable. Thanks for sharing this blog post.