NICE recommends Ikervis in final draft guidance
Posted: 4 November 2015 |
Ikervis has been recommended for the treatment of keratitis in adults with dry eye disease who have not responded to tear substitutes…
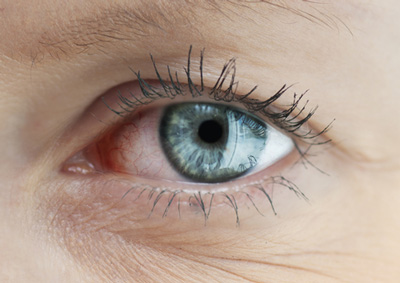

The National Institute for Health and Care Excellence (NICE) has published final draft guidance recommending Ikervis (ciclosporin, Santen Pharmaceutical) for treating severe keratitis in adults with dry eye disease which has not improved despite treatment with artificial tears.
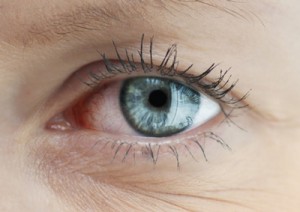

Dry eye disease is chronic inflammation of the eyes caused by reduced tear production or excessive tear evaporation. It can be triggered by a number of factors, including dry or air-conditioned environments, auto-immune diseases (such as rheumatoid arthritis, and lupus), and the adverse effects of some medications. Symptoms include irritation and redness in the eyes, blurred vision, and a sensation of grittiness or a foreign body in the eye. If left untreated, it can result in blindness in severe cases.
Ikervis reduces inflammation in dry eye disease
Ikervis works by reducing inflammation in dry eye disease. However, until the launch of Ikervis, there has been no topical preparation of ciclosporin licensed in the UK. Ikervis has a novel cationic nano-emulsion formulation to effectively deliver the anti-inflammatory potential of ciclosporin. With this recommendation from NICE, Ikervis provides an important new option for the treatment of patients in England and Wales who have not responded to tear substitutes. In October of this year the Scottish Medicines Consortium (SMC) determined that Ikervis should be made available to patients in Scotland.
Professor Carole Longson, Director of the Centre for Health Technology Evaluation at NICE said: “Severe dry eye disease is painful and can have a significant negative effect on day-to-day life for people with the condition. The company responded to the requests in the draft guidance for more information, and we are pleased, therefore, to be able to recommend ciclosporin for adults with severe dry eye disease in final draft guidance published today. I am sure this will be welcome news to patients and healthcare professionals alike.”
NICE expects to publish its final guidance in December 2015.
Related organisations
National Institute for Health and Care Excellence (NICE), Santen Pharmaceutical Co




