Efficient HTS in the nanoliter range
Posted: 22 August 2005 | | No comments yet
The pharmaceutical industry continues to face an ever-changing, increasingly competitive business environment. This makes it imperative for drug discovery and development efforts to incorporate new technologies in order to reduce time-to-market to survive in today’s competitive marketplace. This industry pressure to shorten the R&D process has seen high-throughput screening (HTS) technologies becoming a critical part of the drug discovery process.
The pharmaceutical industry continues to face an ever-changing, increasingly competitive business environment. This makes it imperative for drug discovery and development efforts to incorporate new technologies in order to reduce time-to-market to survive in today’s competitive marketplace. This industry pressure to shorten the R&D process has seen high-throughput screening (HTS) technologies becoming a critical part of the drug discovery process.
The pharmaceutical industry continues to face an ever-changing, increasingly competitive business environment. This makes it imperative for drug discovery and development efforts to incorporate new technologies in order to reduce time-to-market to survive in today’s competitive marketplace. This industry pressure to shorten the R&D process has seen high-throughput screening (HTS) technologies becoming a critical part of the drug discovery process.
HTS technology tests the activity of hundreds of thousands of compounds against a molecular disease target. This activity is a measure of the compound’s ability to inhibit or modify the activity of the target, the first step of a compound to become a potentially promising drug candidate. Until recently, testing of very large compound sets was a bottleneck in the drug discovery process; now, high-throughput screening makes it possible to test more than 200,000 compounds each day – thus making it possible to reduce the R&D cycle. Pharmaceutical and biotech companies are currently implementing and continuing to enhance their HTS initiatives to take advantage of this opportunity to lower attrition rates and ultimately reduce time-to-market.
Depending on the experimental protocol used, HTS assays can provide data that is appropriate for many different purposes: identification of lead compounds, SAR, QSAR and library design. Experimental data may be derived from single concentration screening, testing of a few different concentrations or a complete concentration response curve. In each case, it is important to determine if the experimental protocol will provide the type of data needed for the experimental objective. Replicate experiments determine the variability of the assay and certainly the replicates have to be independent.
It is important to have a good estimate of assay variability, both to characterise the robustness and to identify significant differences between compounds tested in the assay.
Today HTS in the pharmaceutical industry is mainly performed in high density assay plates with 384, 1536 or even 2080 individual measurements per plate. In order to provide all the plates to keep up with the screening throughput and to maintain high quality, automation is required. Automation provides a systematic and repeatable approach for achieving good overall results and prevents plate preparation from becoming a bottleneck in the process.
We at Novartis see efficient HTS with high-quality data content as a key pre-requisite for keeping pace with the ever increasing demands of modern drug discovery and recent developments in genomics research.
The Novartis NanoScreening facility is running HTS studies in highly miniaturised high density plate formats (1536 or 2080 well plates) on two different screening modules1. Total assay volumes range from 0.8 to 6 µl per well or assay data point. This level of assay miniaturisation requires precise compound handling in the low nanoliter range.
For 1536 well screening the compound handling is performed with two compact work cells for sample distribution, formatting and re-formatting in the range of 50 nl to 100 nl2 . They have been developed in collaboration with Zinsser Analytic as an automated compound reformatting module for rapid compound transfer from 384 well source plates into 1536 well assay plates.The systems consist of
- a fast, capillary-based plate replicator and reformatting system for wet and dry plates in the volume range 25nl to 1000nl3
- temperature-controlled storage devices (input stacker for 384 well source plates, output stacker for 1536 well assay plates)4
- a 5-axis robot for the plate handling5
The robot picks up the plates from the storage devices, reads the barcode, places them on the dispense position of the reformatting system and finally brings them back to the port of the storage system. The entire set-up is a stand-alone system in a safety cabinet with easy access from all sides.
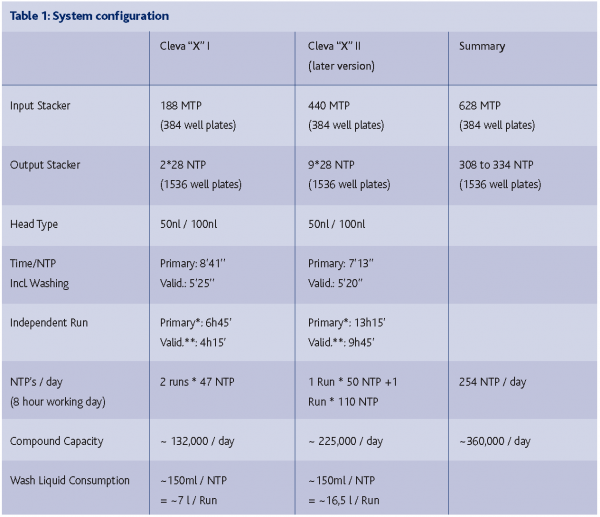
254 assay plates, containing about 360,000 compounds, are generated in this way per day. It takes about eight minutes to run an entire cycle consisting of getting the four source plates and one assay plate from the storage devices, transferring the compound to the 1536 well plate and returning the plates into the storage devices, including washing. (See Table 1: System Configuration below, validity time). Less than 5 and a half minutes are required for the reformatting step.
Due to the high speed of operation, the assay plates containing the compounds can be generated on demand and ad hoc. The working range of compound reformatting from 384 well compound source plates is 20, 25, and 100nl. The unattended operation of one module can achieve the reformatting of more than 150,000 compounds in 13 hours. This strategy allows screening of freshly reformatted compounds.
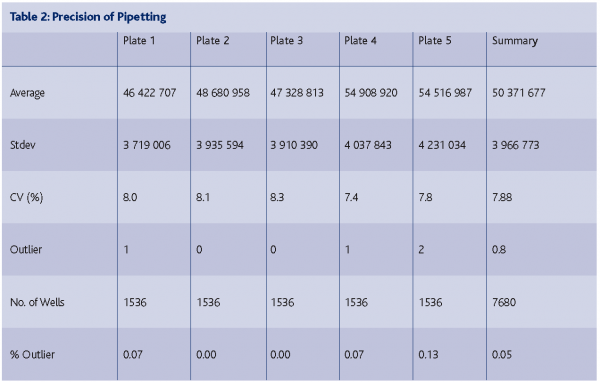
The systems transfer the compounds in the low nanoliter range very reliably with very low outlier rates (< 0.1 %) and CVs (coefficient of variation < 8 %). For different volumes a variety of dispensing heads are available for the reformatting system. So far we are working with the 50 and 100 nl volume dispensing heads. Outliers can occur if one of the capillaries has been blocked. The development of efficient pipette washing protocols has brought the well-to-well cross contamination rate below 0.1% (see Table 2: Precision of Pipetting below)
In conclusion, with our reformatting module we have a powerful, fast and reliable tool to transfer nanoliter amounts of screening compounds into 1536 well assay plates. The compound handling steps are fast enough to enable screening of freshly reformatted compounds and the performance as well as the reliability of data, which are of very high quality. The performance of the module has convinced us to use the Cleva the systems for IC50 determination processes as well.


References
- Evoscreen Mark-II and Evoscreen Mark-III by Evotec OAI, Ltd. Abingdon, Great Britain
- CLEVA “X” by Zinsser Analytic GmbH, Frankfurt, Germany
- HummingBird by GenomicSolutions, Huntingdon, Great Britain
- by Liconic AG, Mauren, Liechtenstein
- by Thermo Electron Cooperation, Dreieich, Germany




