The current state of PAT in freeze drying
Posted: 11 November 2005 | | No comments yet
Freeze drying is a widely used method to stabilise protein pharmaceuticals. The stability of proteins and the biological activity can be influenced by several factors, which may lead to conformational changes and to denaturation, aggregation or absorption to surfaces1.
To develop a formulation that is stable for up to two years is challenging. Aqueous, ready-to-use solutions are preferable dosage forms (convenience, cost, customer acceptance), but often insufficient stability in solution can not be overcome. Therefore, many protein dosage forms are produced by freeze drying. Freeze drying comprises three different stages, beginning with the freezing procedure followed by the primary drying and finally the secondary drying step. In order to develop an economical and sustainable freeze drying cycle, it is crucial to minimise the process time, especially for primary drying. The ice sublimation rate is the most important factor within this process step2. To stabilise proteins against denaturation stress, a variety of basic principles can be applied. These measures include adjusting the pH and buffer system, the addition of cryoprotectants and the selection of lyprotectants to create amorphous matrices with high Tg and water replacement properties. A residual moisture content below 2% must be reached and maintained during storage3,4. Accordingly, when amorphous matrices shall be dried, the main drying temperature must be kept very low which consequently leads to longer drying cycles. Latter cycles increase the need for cycle time optimisation with modern monitoring tools; the process analytical technologies (PAT). PAT systems include fundamental process design and optimisation, as well as control devices but also statistical response strategies and other measures. After such techniques have been adapted and approved, the quality level of products can be maintained in a very narrow range; time consuming analytical measurements may be reduced, or even cancelled for the end products, finally leading towards parametric release concepts.
Process monitoring methods
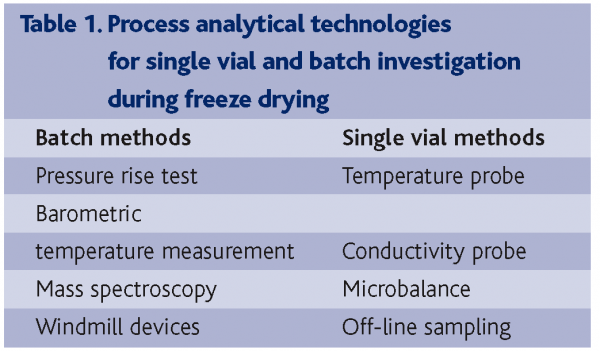
For freeze drying where the process development is a critical preparation step, process analytical technology is a mandatory part of the entire quality system. Freeze drying of a particular formulation requires the optimisation of numerous parameters as product presentation (e.g. volume, packaging material) and formulation dictate the process conditions. Process monitoring methods can be divided into two groups. The first group comprises temperature probe, conductivity probes, balances and offline analytics after sampling via the sampling theft. The second group is formed by all methods that monitor the entire batch – not single vials – and barometric temperature measurement, pressure rise test, windmill devices, mass spectroscopy and moisture sensors have to be named here (see Table 1). The pros and cons of all these methods are reported in the literature. In the following, some major issues will be exemplified. By using temperature and electrical resistance probes, conditions in selected samples can be monitored in a rather precise and meaningful way. However, irritations and complications connected to the latter methods are well known. For example, temperature probes can serve as a crystallisation point during freezing and strongly affect heat conduction in the drying cake5. This invasive method, however, can still be entitled as the gold standard for process development. Noninvasive methods, including the pressure-rise test6 and the barometric temperature measurement7, involve short interruption of the drying cycle with the associated danger of partial ice-melting or product collapse. Furthermore, these methods are highly dependent on the leak rate of the equipment and therefore very sensitive to maintenance issues. Comparative pressure measurement using Pirani sensors and capacitance manometers provide quantitative information, but require repeated calibration8. In fact, this method only delivers indirect information about the end of primary drying. The lack of new methods to characterise the true situation inside freeze drying processes relates to their extensive experimental set up. Yet the combination of such tools could finally increase the understanding of manufacturing processes and is therefore of high value. Novel methods to determine the status of lyophilized formulations online are the microbalance system, near-infrared-spectroscopy (NIRS) and mass spectroscopy.

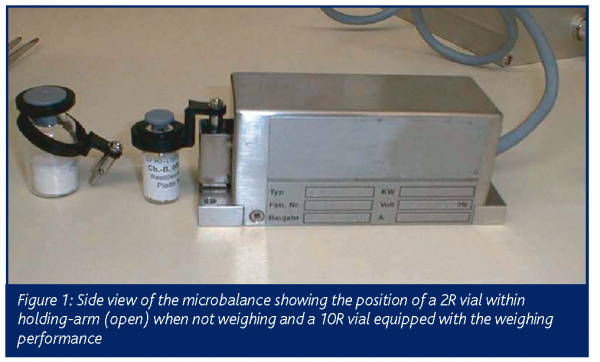
Microbalance system
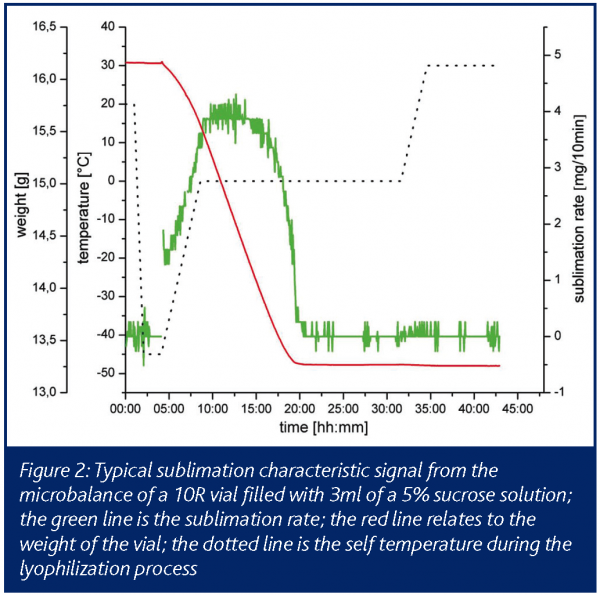
The microbalance system allows the direct online monitoring of the primary drying process in terms of mass loss of water/solvent. In recent years, a microbalance for monitoring the freeze drying cycle has become commercially available. The device used in this study allows continuous weight determination of vials of the sizes 2R and 10R, whereby the microbalance itself is located on the shelf of the drying chamber. With a measuring range of 50g, a resolution of 5mg and a working temperature down to minus 40°C, the method can be applied in a relevant working range. The operation principles can be described as follows: one vial, filled with product solution, is placed on the shelf within the holding arm (Figure 1). At pre-programmed weighing time intervals, the holding arm lifts the vial. This lifting and weighing step takes approximately 10s, after which time the vial is released from the holding arm. Continuous monitoring of cumulative water loss and the temporary drying rate can be used for the comparison of packaging material, the determination of process conditions and for formulation studies. Basically, the shape of the drying curve is influenced by the process conditions and the cake forming agents themselves (Figure 2). Thereby, significant differences could be shown in relation to the used cake forming agents and the primary drying conditions9. The drying rate curves can point out differences in shelf temperature or chamber pressure. Due to the direct determination of the sublimation rate the microbalance system is superior to other online systems. Compared with ordinary temperature sensors a direct signal for the sublimation rate, not a surrogate parameter, can be detected.
However, the impact of thermal energy input by the balance itself on the drying speed and the non-sterility must be taken into account. Nevertheless, the microbalance can be used as a favourable tool for the end point determination of primary drying.


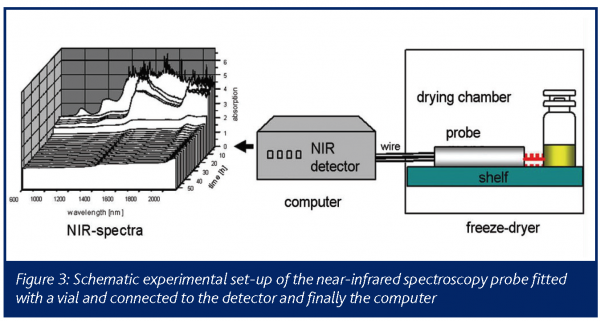
Near-Infrared Spectroscopy (NIRS)
NIRS allows a non-invasive, non-destructive and rapid moisture determination and offers a wide range of capabilities in the process analytical technology of freeze drying pharmaceuticals10. The end of the primary and secondary drying process can be precisely determined. Moreover, the freezing point, the ice formation process and the transition from frozen solution to dried material can be observed.
The major advantage of this method is the simultaneous and rapid insitu process monitoring for process and product conditions resulting in the direct determination of the moisture during different dying stages11. Other existing analytical techniques for direct moisture detection within a formulation matrix work exclusively off line. Compared to methods such as X-ray powder diffraction12 or Infrared spectroscopy13, NIR spectroscopy is able to study product characteristics inside a conventional lyophilizer online. An NIR-system model 6500 online spectrometer was used to collect the near infrared reflectance spectra. Partial least squares (PLS) regression was used to form the relation between the residual moisture content and the near infrared reflectance spectra as obtained for the 128 samples that were analysed. Therefore, the NIR sensor is located on the shelf inside the drying chamber and directly fitted with the probe tube to the outer wall of the vial (Figure 3). This arrangement offers the advantages of sterility compared to other set-ups, where the NIR probe is located inside the vial in combination with a thermocouple14. It is furthermore much less disturbing as it does not affect the drying kinetics of the sample vial, nor does it restrict the fill volume, vial type etc. An optimal calibration model with a number of PLS factors was obtained.

In comparison to Karl-Fischer data and temperature probes an excellent correlation is presented up to a residual moisture content of approximately 4%, with a very low standard deviation of 0.2%. The correlation coefficient, NIRS vs. Karl-Fischer was 0.99 (Figure 4).
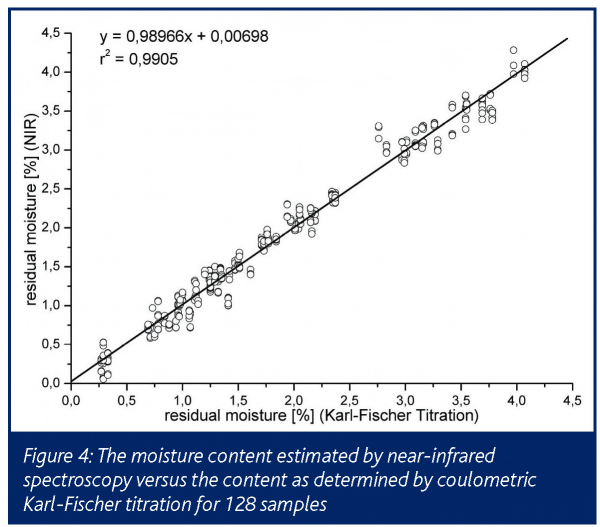

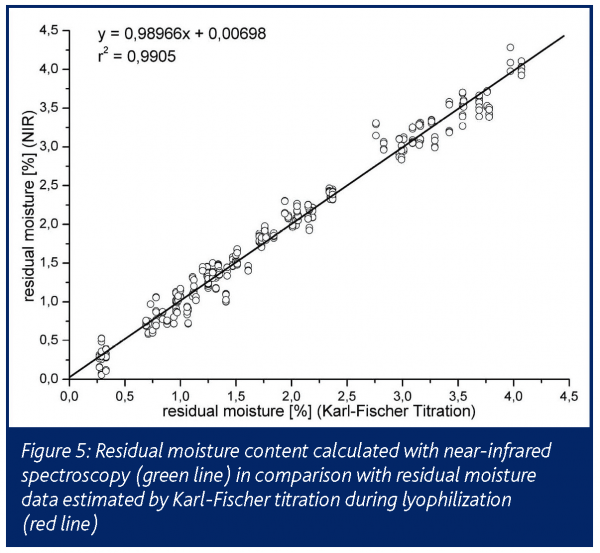
The direct detection of the residual moisture during primary drying and the precise detection within secondary drying correlated well with the results obtained from other methods (Figure 5). It must be noted that the residual moisture of the sample vial is always underestimated by NIR compared to offline Karl-Fischer assays15.
The incomplete penetration of the NIR-waves into the innermost core of the sample vial must be assumed as the reason for that shift. The system is rather expensive and requires elaborate calibration when applied to a new formulation. On the other hand, it delivers significantly more product and process information than any other method directed to online residual moisture detection.

Mass spectroscopy
The implementation of mass spectroscopy in lyophilization allows the direct control of the partial pressure of vaporized water during primary and secondary drying. The main advantage of this analytical method is to monitor the process without interfering with the drying process and the product at all. The application of the mass spectroscopy to freeze drying systems was proposed many years ago but has not been applied in the pharmaceutical industries since then16.
The arrangement used in this study was a portable spectrometer. The sample port for representative measurements was located on the vacuum line between the drying chamber of a common freeze dryer and the condenser (Figure 6). To ensure a fast response, the vacuum tube was preheated up to 120°C to avoid interference inside the spectra.
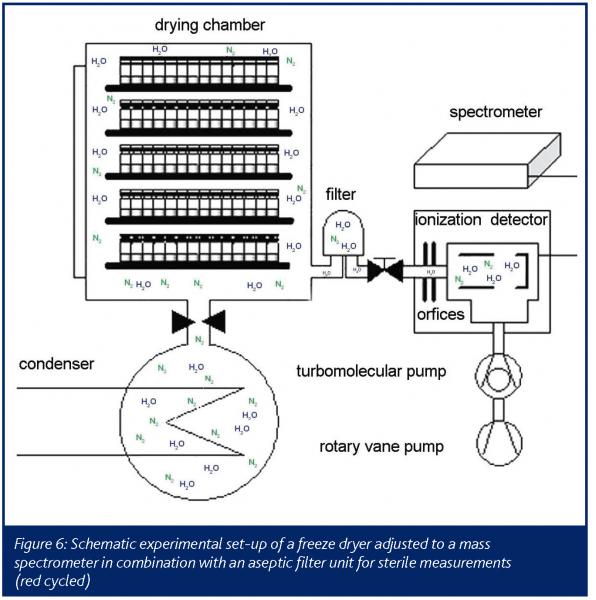

The required vacuum inside the ionization chamber was obtained by coupling several pumps in series, to achieve a constant pressure gradient. The detection of water during primary drying was started when the present vacuum inside the drying chamber was reached by opening two orifices in the connective tube. The detection of water vapour and nitrogen was conducted in 30s detection intervals. When opening the orifices, the signal rises almost immediately showing the spectrometers fast response. After reaching primary drying temperature the plateau region of the signal is visible. Water vapour is continuously sublimated from the ice and condensed on the low temperature surfaces in the condenser chamber. Endpoint of the sublimation and of the primary drying was apparent due to a rapid decrease of the water vapour level inside the drying chamber. The end of the sublimation phase is easily detectable and secondary drying can be started by increasing the shelf temperature. In contrast to the situation with temperature probes and balance systems, it can be assumed that mass spectrometry reflects also worst case samples which finally determine the water vapour output and the operator is safely prevented from increasing shelf temperature too early, risking immediate collapse or melt back. The increased energy input from the heated shelves then leads to a second water vapour peak until the diffusion of water from the dry cake finally levels off (Figure 7). This results in residual moisture data between 1 and 2%. The endpoint of the secondary drying is characterised by a baseline value with no water left in the product chamber (Figure 7). The offline data for residual moisture (Karl-Fischer) and the mass spectroscopy data correlate very well during the late phase of primary drying and over the secondary drying.
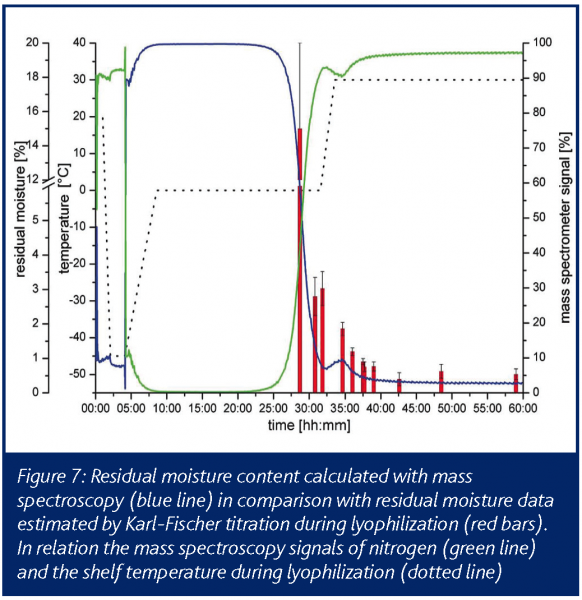

To verify the endpoint determination and the partial pressure of water vapour during secondary drying, different formulations were studied with similar outcomes. Furthermore, the direct comparison and correlation with pressure measurement using Pirani sensors and temperature probes suggested mass spectroscopy as an encouraging system for endpoint determination in primary drying with added benefit for secondary drying where the other methods show no effect.
The possibility to shorten the primary drying process for several hours by analysis of the mass spectroscopy curves could be demonstrated. In addition, only slight differences in the signal of the mass spectrometer by varying the loading capacities of the freeze dryer from 1/3 to full load were obtained. With implementation of an aseptic sterile filter between drying chamber and the spectrometer, a remarkable application base can be proposed today. It is possible to apply this valuable method not only in development processes but also in commercial scale production setups where full aseptic conditions are mandatory. The method delivers a clear signal for the end of primary and secondary drying, is not compromised by single vial effects or other distributing features and by the use of validated sterile gas filters can be considered as sterile.
Remarks
The application of novel PATs in freeze drying is a very important step towards more understanding of the processes and (especially in light of today’s protein drug formulation) a measure to optimise such processes in terms of time and failure rate. The microbalance system offers a favourable tool for online monitoring of primary drying during research and development purposes. However, this tool can only be used in development, due to its non sterility and process interference.
Mass spectroscopy can be recommended as a favourable method for development and routine production, where it is possible to use its ability of exact end point determination of the primary and secondary drying stages within a fully aseptic environment. Despite considerable costs, a portable mass spectrometer fulfils the demands and can be adapted to more than one dryer if needed. NIR spectroscopy is the only method delivering direct online data about the moisture within a particular formulation. We propose a setup that is advantageous over previous approaches due to its rather non-invasive sensor and wide applicability. However, with the necessity for extensive calibration and the limited penetration depth of the NIR beam, the method is not yet optimised.
Acknowledgement
This article originally appeared in American Pharmaceutical Review January/February 2005.
References
- Arakawa, T., Prestrelski, S.J., Kenney, W.C., Carpenter, J.F., Factors affecting short-term and long term stabilities of proteins, Adv. Drug Deliv. Rev., 10: 1-29 (1993).
- Pikal, M.J., Shah, S., The collapse temperature in freeze drying: dependence on measurement methodology and rate of water removal from the glassy phase, Int. J. Pharm., 62 (2-3): 165-186 (1990).
- Wang, W., Lyophilization and development of solid protein pharmaceuticals, Int. J. Pharm, 203: 1-60 (2000).
- Tang, X., Pikal, M.J., Design of freeze drying for pharmaceuticals: practical advice, Pharm. Res., 21 (2): 191-200 (2004).
- Roth, C., Winter, G., Lee, G., Continuous measurement of drying rate of crystalline and amorphous systems during freeze drying using in situ microbalance technique, J. Pharm. Sci., 90 (9): 1345-1355 (2001).
- Nail, S., Johnson, J., Methology for in-process determination of residual moisture in freeze dried products. Devel. Biol. Std., 74: 137-151 (1992).
- Milton, N., Pikal, M., Roy, M., Nail, S., Evaluation of manometric temperature measurement of a method for monitoring product temperature during lyophilization, J. Pharm. Sci. Technol., 51: 7-16 (1997).
- Jitschin, W., Ludwig, S., Dynamical behavior of the Pirani sensor, Vacuum, 75: 169-176 (2004).
- Presser, I., Denkinger, N., Hoermann, H., Winter, G., New methods in the monitoring of freeze drying processes: Validation of the microbalance, Central European Symposium Pharmaceutical Technology Conference, Vienna (2001).
- Ciruczak, E.W., Growth of near-infrared spectroscopy in pharmaceutical and medical sciences, A. Pharm. Rev., 5: 68-73 (2002).
- Presser, I., Denkinger, N., Hoermann, H., Winter, G., New methods in monitoring of freeze drying: near infrared spectroscopy determination of residue moisture during freeze drying, Protein Stability Conference, Breckenridge, Colorado (2002).
- Cavatur, R.K., Suryanarayanan, R., Characterization of phase transitions during freeze drying by in situ X-ray powder diffraction, Pharm. Dev. Technol., 3: 579-568 (1998).
- Remmele, R.L., Stunshnoff, C., Carpenter, J.F., Real-time in situ monitoring of lysozyme during lyophilization using infrared spectroscopy – dehydration stress in the presence of sucrose, Pharm. Res., 14: 1548-1555 (1997).
- Bruells, M., Folestad, S., Spáren, A., Rasmuson, A., In-situ Near-Infrared spectroscopy monitoring of the lyophilization process, Pharm. Res., 20 (3): 494-499 (2003).
- MacDonald, B.F., Prebbe, K.A., Some applications of nearinfrared reflectance analysis in the pharmaceutical industry, J. Pharm. Anal., 11: 1077-1085 (1993).
- Connelly, J.P., Welch, J.V., Monitor lyophilization with mass spectrometer gas analysis, J. Parenter. Sci. Technol., 47: 70-75 (1990).
Issue
Related topics
Freeze Drying, Lyophilisation, Process Analytical Technologies (PAT)





