Investigational plaque psoriasis treatments show promise in late-stage trials
Posted: 11 March 2025 | Catherine Eckford (European Pharmaceutical Review) | No comments yet
With the Phase III trials demonstrating sustained skin clearance in plaque psoriasis, this could lead to patients accessing more treatment options to manage the autoimmune inflammatory disease.
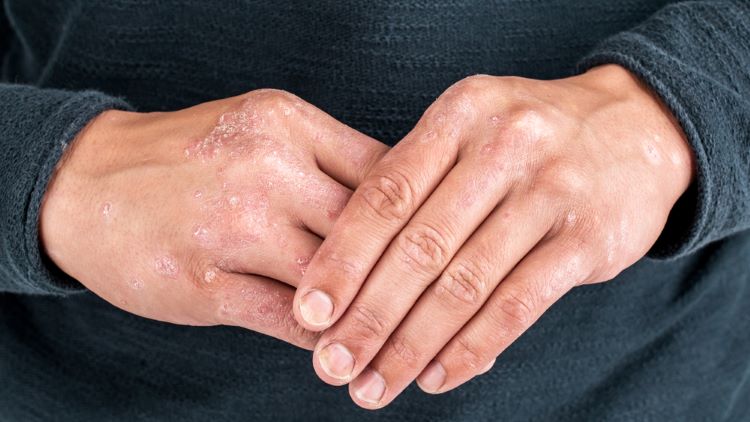

Newly released Phase III data illustrate potential of three drugs being investigated as treatments for plaque psoriasis—namely two monoclonal antibodies and an oral peptide, from pharmaceutical companies Biocon Biologics, UCB and Johnson & Johnson, respectively.
Firstly, recent data from Biocon Biologics’ Phase III trial show that its monoclonal antibody YESINTEK “offers an effective, safe, and comparable alternative to reference [biosimilar] Ustekinumab in the treatment of moderate to severe chronic plaque psoriasis and by extension the other indications for which Ustekinumab is indicated,” explained Dr Uwe Gudat, Chief Medical Officer, Biocon Biologics. The data was presented at the 2025 American Academy of Dermatology (AAD) Annual Meeting.
UCB’s monoclonal antibody for plaque psoriasis
Long-term findings from the BE BRIGHT study showed that over a five-year period, of a subset of patients from the second extension of the trial, 67.7 percent given with BIMZELX® (bimekizumab-bkzx) achieved PASI100, representing sustained complete skin clearance.
BIMZELX is the first and only approved medicine designed to selectively inhibit both interleukin 17A IL-17A and IL-17F.
“These five-year results highlight the robust potential of bimekizumab-bkzx in transforming patient outcomes by offering the possibility of lasting, complete skin clearance… our belief in its innovative dual inhibition approach is reflected in our dedication to head-to-head trials,” shared Fiona du Monceau, Executive Vice President, Head of Patient Evidence, UCB.
This data for BIMZELX was presented at the 2025 American Academy of Dermatology (AAD) Annual Meeting.
Johnson & Johnson’s oral peptide plaque psoriasis treatment
Johnson & Johnson’s icotrokinra (JNJ-2113) demonstrated robust skin clearance in participants with plaque psoriasis in the Phase III ICONIC-LEAD trial, the company has shared.
“These study results are promising, and show the potential for treatment with icotrokinra to offer patients the unique combination of complete skin clearance and a favourable safety profile in a once daily pill”
Forty six percent of patients with moderate-to-severe plaque psoriasis treated with investigational icotrokinra attained complete skin clearance (IGA 0) at Week 24. Additionally, 40 percent of participants reached PASI 100.
These results lead the way to the start of the first head-to-head study intended to demonstrate superiority of a pill versus injectable biologic in moderate-to-severe plaque psoriasis, according to Johnson & Johnson.
“These study results are promising, and show the potential for treatment with icotrokinra to offer patients the unique combination of complete skin clearance and a favourable safety profile in a once daily pill,” stated Dr Robert Bissonnette, Chairman at Innovaderm Research, Montreal, Canada and ICONIC-LEAD study investigator.
“…we are proud to advance this first-in-class investigational targeted oral peptide that selectively blocks the IL-23 receptor, which shows promise as a potential first-line systemic therapy for the treatment of plaque psoriasis,” remarked Liza O’Dowd, Vice President, Immunodermatology Disease Area Lead, Johnson & Johnson Innovative Medicine.
Related topics
Big Pharma, Clinical Development, Clinical Trials, Drug Development, Drug Safety, Industry Insight, Research & Development (R&D), Therapeutics
Related organisations
Related drugs
Bimzelx, biologic, Biosimilars, icotrokinra, monoclonal antibodies (mAbs), oral drugs, Ustekinumab, YESINTEK
Related people
Dr Robert Bissonnette, Dr Uwe Gudat, Fiona du Monceau, Liza O'Dowd









