Specialised microbial testing is a vital part of routine environmental monitoring, analysis of critical findings and the drug development strategy. Throughout the development and manufacturing processes, the potential for bacterial and fungal contamination of raw materials, operator clothing, production facilities and finished products poses a serious threat to drug efficacy and patient safety – and can place a significant time and cost burden on producers. In this highly regulated sector, manufacturers must establish quick and robust environmental monitoring and contamination control strategies as part of current good manufacturing practice (cGMP).
MPL in Innsbruck, Austria, offers validated microbiological analysis and release testing for the pharmaceutical industry, conducted according to the current regulations defined by the European Pharmacopeia (EP), international ISO standards, the United States Pharmacopeia (USP) and by the European Medicines Agency (EMA), all within the cGMP umbrella. Recent adoption of Bruker’s matrix assisted laser desorption/ionisation-time of flight mass spectrometry (MALDI-TOF MS) technology for microbiology labs has dramatically increased throughput, while maintaining the high-quality service, regulatory compliance and accurate results demanded by its pharmaceutical client base.
Quality control: a time-consuming process
The need for rapid, accurate and validated microbial detection and identification is clear, particularly in pharmaceutical product release testing where delays can cause costs to spiral. When bacterial or fungal isolates are found in a production facility, it is essential to identify the organism rapidly and reliably at species level. Accurate identification supports tracking, trend reporting, and setting up a reliable data log to inform future preventative methods.
Traditional plating has long been considered the gold standard in microbial identification. Genomic techniques are considered the new best rapid solution in microbial identification. This approach is based on polymerase chain reaction (PCR) and deoxyribonucleic acid (DNA) or ribosomal ribonucleic acid (rRNA) sequencing for monitoring, detection and identification of potential contaminants, to ensure raw materials are compliant to their specification. Analysing sequence data, however, is complex and requires technical expertise, plus the performance of different sequencing identification systems varies, takes time and is comparably costly. The implementation and validation of a sequencing system in a cGMP environment presents a challenge.
Sequencing is also time consuming. In this time-critical, high-cost environment, a better solution at a comparable quality level is needed that offers the same high level of data quality and data integrity, but faster.
Presenting the next generation of microbial testing
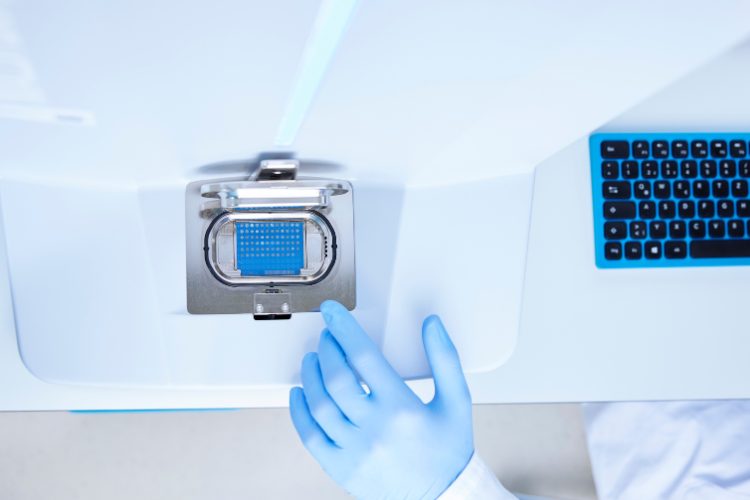
MPL’s new flagship microbial identification system is based on MALDI-TOF MS, which has dramatically accelerated throughput in the laboratory without compromising performance. The Bruker MALDI Biotyper (MBT) sirius is recognised as a fast, reliable and reproducible microbiology method for microorganism identification.
The MBT workflow delivers validated results within minutes from a small amount of culture. The quality of identification plus its speed, ability to run multiple sample tests in parallel, and to repeat samples in short times, has brought down identification time to around two minutes per sample, representing a valuable turnaround time saving compared to the previous throughput of up to two days via sequencing. The system is flexible for medium or high-throughput workloads, and is further enhanced at MPL by the world leading and validated Accugenix® reference database of more than 16,000 entries for bacteria and fungi, made up of Bruker’s high-quality library and the significant Charles River in-house library, which together ensure reliable and accurate results.
Supporting product release testing
The system is used for customer samples from both the sterile and non-sterile environment. In the sterile manufacturing line, pharmaceutical and biopharmaceutical manufacturers need to confirm whether the product is free of microorganisms throughout the manufacturing process, as contamination can be introduced at different stages. At the final stage, the bioburden test is conducted before the sterilisation process to see if any potential contaminant is present in the sample – for example, fungal spores or bacterial growth. From the microbiological perspective, this allows the opportunity to establish any issues and propose improvements, directly at production stage.
In non-sterile manufacturing, a typical specification for a pharmaceutical product will be to confirm the absence of potentially harmful pathogens such as Escherichia coli or Salmonella. It is vital that the analysis returns a confident and accurate result.
Another fast growth area of microorganism identification in the pharmaceutical industry is the field of environmental monitoring. Pharmaceutical manufacturing falls under the EU GMP Annex 1 guideline, which requires environmental monitoring programmes where samples are cultured and analysed. Microorganism identification of the pharma microbiome per product and per production line is a critical part of that, especially for cleanrooms grade A/B, but also lower grade critical areas.
Fungal identification
While fungal contamination is less common than bacterial contamination in terms of customer sample numbers, it can have a major impact on the manufacturing process, the product and the patient. Identifying moulds and multicellular fungi is one of the most challenging aspects of microbiology, and fungal contamination is under increased scrutiny as contaminated pharmaceuticals could lead to outbreaks of severe fungal diseases in the community.3 Filamentous fungi, in particular, are notably difficult to identify, and in many laboratories across the globe this is a fully manual – and therefore subjective – process. The MALDI-TOF workflow no longer requires liquid cultures, as Bruker has introduced the Mycelium Transfer (MyT) procedure with easy direct fungi transfer.
The Bruker MBT HT Filamentous Fungi Module has been created to enable rapid identification of challenging filamentous fungi at species level, through isolation of mycelium. The workflow introduces a standardised approach, combining an extensive reference library with advanced software that supports automation of mass spectral acquisition and analysis to deliver accurate identification. The world of fungi is large, and the customer can constantly expand their own database or with the help of Charles River.
Conclusion
The pharmaceutical industry is set to see continued globalisation of outsourcing, as samples are tested by the selected laboratory service partner regardless of location, to ensure a standardised approach. State-ofthe- art technology featuring a high degree of automation, consistent analysis and high throughput is set to lower costs and increase efficiencies to bring safe and effective drugs to market more quickly.
MPL – Mikrobiologisches Prüflabor GmbH
Since the early 1990s, MPL – Mikrobiologisches Prüflabor GmbH (MPL) has offered microbiological laboratory services to clients, initially locally and expanding internationally to become an integral part of the global pharmaceutical service sector. The team combines decades of experience in scientific product release testing with a spirit of innovation and customer centricity that continues to drive the business forward.
FOR SPECIALIST SERVICE LABORATORY CONTACT MPL – www.mpl.co.at
Bruker Microbiology & Infection Diagnostics
Bruker Microbiology & Infection Diagnostics supports the pharmaceutical industry in the rapid identification and analysis of microorganisms. The intuitive, robust instrumentation and streamlined workflows meet the needs of microbiologists in clinical and industrial.
FOR MORE INFORMATION, CONTACT BRUKER – www.bruker.com
References
- Statista: Pharmaceutical products and market, September 2023. https://www.statista.com/statistics/1414301/outsourcing-drug-developmentactivities-by-companies/
- Deloitte: Drop-off in returns on R&D investments, January 2024. https://www2.deloitte.com/ch/en/pages/press-releases/articles/deloittepharma-study-drop-off-in-returns-on-r-and-dinvestments-sharp-decline-in-peak-sales-per-asset.html
- Ahmed MAEE, Abbas HS, Kotakonda M. Fungal Diseases Caused by Serious Contamination of Pharmaceuticals and Medical Devices, and Rapid Fungal Detection Using Nano-Diagnostic Tools: A Critical Review. Curr Microbiol. 2023 Nov 17;81(1):10. doi: https://doi.org/10.1007/s00284-023-03506-7. PMID: 37978091; PMCID: PMC10656328.
For further information, visit: www.bruker.com/microbiology