Challenges in pharmaceutical microbiology: looking to the future
Posted: 23 February 2023 | Edward C Tidswell (Merck & Co Inc.) | 1 comment
Here, Edward C Tidswell, Executive Director Quality Assurance at Merck & Co, Inc., discusses the microbiology challenges for industry, pharmaceutical companies and microbiologists themselves.


Pharmaceutical microbiologists are subordinate to the firms they are employed by, so usually a firm’s challenges – and how they choose to address them – will dictate the challenges of its employees. Although there is some overlap between the challenges relevant to firms and to their microbiologists, there is some attendant level of misalignment that does need frank recognition, robust discussion and which necessarily demands reconciliation.
Despite this overlap, this article addresses the main challenges for industry and firms, and those for pharmaceutical microbiologists, separately, focusing on some of the difficult and often overlooked hurdles relating to quality assurance (QA) and quality control (QC) of licensed and marketed pharmaceutical drug products. Given the widely reported challenges of validation and qualification of microbiological methods, as well as lab compliance and keeping up with compendial amendments, I have steered away from these topics here.
These are solely my personal opinions derived from recent and 25 years of experience in the pharmaceutical industry, supporting firms, microbiologists, industry organisations, compendia and standard-setting bodies. I would add that these do not necessarily represent the opinions of those organisations I support or work for.
Pharmaceutical microbiology: challenges for industry
Automation
It is not uncommon for QC microbiology laboratories to include analytical instrumentation that incorporate a level of automation. It is often used for sample handling, dilution, analysis and evaluation. Continued implementation across industry of such instrumentation is essential for the economic fitness of our sector and the assurance of analyses consistently performed to standard operating procedure, giving greater confidence in the data derived therefrom.
Today there are several options available for automated colony counters incorporating the physical handling, incubation and enumeration of colonies. However, firms must establish if or how these platforms diverge from compendial methods and possible failure modes. Failing to do so will inevitably lead to non-compliance risks and may hinder operation of the platform. Firms will need instrument vendors to expand their portfolio of equipment to accommodate a broader range of analyses and tests performed in the pharmaceutical QC laboratory.


The difficulty today and always is that pharmaceutical microbiology is a demanding vocation frequently misunderstood and seldom recognised for its inherent challenges.
Currently entering the market are ‘robotic’ systems that perform environmental monitoring, cleaning and disinfection processes. These offer the advantages of controlling bioburden entering a cleanroom, cleanroom control and the assurance of sustained, consistent and compliant practices. Is this really the zenith of contamination control innovation? I suspect not and look forward to the eventual retirement of the pharmaceutical cleanroom. Nevertheless, firms must carefully assess the utility of these systems and how they currently integrate with human operators, operations and personnel. This may represent an area where regulatory requirements could be swiftly developed with dual impact to industry and health authorities.
Far more important than individual analytical platforms in the QC microbiology laboratory is the development and implementation of sample handling systems that operate end-to-end. Ideally, this would entail the automated transfer of samples from their origin through delivery to the laboratory, sample storage, handling, automated analytics and finally disposal. Such an operationally integrated system would provide huge benefit in terms of chain of custody, sample control, analytics and data integrity in addition to the significant benefit of genuinely removing headcount from the laboratory. Such systems are already partially or fully available across other industry sectors and firms will need vendors to expand their portfolio.
Firms across the pharmaceutical industry must collectively encourage and demand availability of these forms of instrumentation and equipment. Regulatory agencies and health authorities may start to encourage greater end-to-end automation in the near future.
Digital system integration
Complete and effective integration of digital solutions in the microbiology laboratory interfaced with local and enterprise-wide information management systems must be accomplished. Technology is developing exponentially, yet digital component systems (electronic notebooks, automated testing platforms, novel non-compendial test methods and laboratory information systems) are not always easily or sleekly interfaced to provide a truly seamless experience.
In the future, the pharmaceutical industry will need to grapple with applying deep learning, machine learning and artificial intelligence to the realm of QC microbiology
For example, configurations of information systems exist that do not permit or easily permit the use of power notations such as 102. This illustrates the sometimes-vast misalignment between microbiology and technological solutions. There are diverse options in terms of novel testing platforms, including those alternatives to compendial microbiological methods supplied from a diverging array of vendors. These frequently fail to integrate well with legacy information and execution systems. Rapid microbiological testing platforms are an example where historically complete and full digital integration with legacy systems is not always straightforward. Ideally a single supply of end-to‑end automated systems and associated local and enterprise-wide information management would be most desirable for firms.
Knowledge management, data and artificial intelligence systems
The breadth and depth of knowledge associated with microbiology and pharmaceutical microbiology is growing exponentially (Figure 1) and will continue to. Generally, however, the field of microbiology is suffering ‘growing pains’ whereby the volume of literature, research/author bias and increasing specialisation is limiting advancement.1 Concurrently, the field of microbial systematics continues to change. Pharmaceutical microbiologists rely upon genuinely representative citation information, interpretation and conclusion to support an informed analysis of data. All these data and information must be readily available coupled with a firm’s own data from microbiological studies and analysis. Firms will need to ensure appropriate and evolving platforms in knowledge management are available.
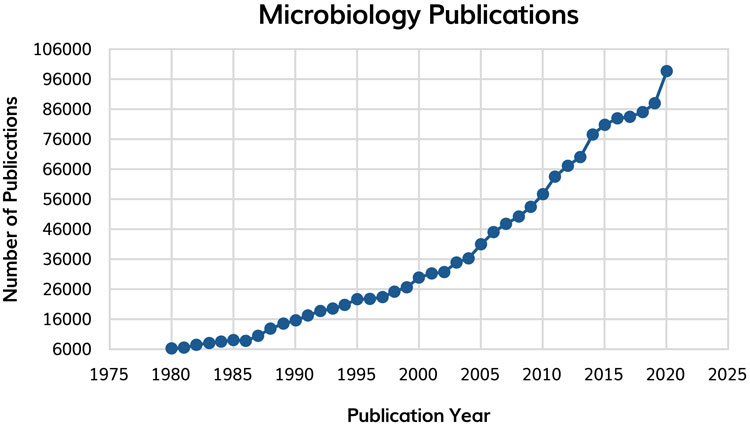

Figure 1: The exponential increase in microbiology publications 1980 – 2020. Data were obtained from PubMed searching for ‘Microbiology’. The number of publications in 2021 and 2022 decrease slightly, likely due to the impact of COVID.
In the future, the pharmaceutical industry will need to grapple with applying deep learning, machine learning and artificial intelligence to the realm of QC microbiology. These technologies have and are being successfully applied to microbiome studies, taxonomy, microbial ecology, pathogenicity, epidemiology and antimicrobial peptide discovery.2 As microbiological data (quantitative and species data) are accrued from testing, process and environmental monitoring analysis is better suited to automated non-human systems. An example of a simple application currently available is the use of computerised flow dynamics (CFD) in the accurate modelling and prediction of airflows in traffic‑bound and traffic-free cleanrooms and isolators. Applying CFD permits a quantitative and accurate determination of microbial and particulate distributions and various risks in controlled environments. This removes the current practices of minimal sampling, assumption-based quality risk assessments and human experience in the establishment of environmental monitoring programmes and the process/product impact contamination risk assessments.
Recruitment challenges
The recruitment of career microbiologists remains problematic. This challenge has been accentuated by COVID and post-COVID changes in career expectations. It is possible that this will usher in a completely new means of managing pharmaceutical microbiology wherein automation is augmented with artificial narrow intelligence, or artificial general intelligence, to almost completely remove microbiology scientists from the laboratory.
Pharmaceutical microbiology: challenges facing pharmaceutical microbiologists
Managing traditional compendial method data with alternative method data
Compendial microbiological methods are regarded as the ‘referee method’. In other words, where there is any question or dubiety concerning the microbiological quality attribute of a test sample, compendial methods are the deciding analytical tests and procedures. Most microbiological compendial methods are based upon traditional methods which provide an analytical result that permits the user to assess the test sample’s quality attribute against those stipulated in other compendial chapters or content. For example, USP <61> is the test method used to ensure non-sterile dosage forms comport to the levels of microorganisms required by USP <1111>. Both USP <61> and USP <1111> define the maximum permissible quantities of microorganisms as the colony forming unit (CFU). A similar principle applies to a test for sterility and a claim of sterility. Herein lies the issue: many alternative (modern) microbiological methods do not report results in colony forming units (CFU) and their units of measure do not always convert with accurate proportional uniformity along a calibration curve. The compendia do not appear to be making significant changes to move away from the CFU as the diagnostic of microbiological quality and it continues to be left to firms and pharmaceutical microbiologists to manage this.
Knowledge management
Until industry and firms in general optimise knowledge management systems the pharmaceutical microbiologist will continue to struggle in this domain. Microbiologists will continue to rely upon availability of time and their own know-how in searching and sifting through published articles. This is an especially important task when assessing if a microorganism is regarded as objectionable and if recovery of a particular type of microorganism from a particular location/sample represents a risk.
Technical competency and career
Insightful and innovative pharmaceutical firms will increasingly leverage the expertise of career pharmaceutical microbiologists. Career microbiologists are well recognised as fastidious, possessing a level of expertise and initiative demanding of the field. Pharmaceutical microbiology continues to require microbiologists with an aptitude for managing variability and ambiguity, which is historically associated with analytics and data analysis. As such, microbiologists will need to work with increasing frequency in the manufacturing space as recent compliance guidance (eg, Annex 1) demands firms have cohesive contamination control strategies. Careers in pharmaceutical microbiology will likely transition over the next few years due to firms outsourcing typical microbiological tasks, increasing automation, digitalisation and the increasing emphasis on improved manufacturing controls and contamination control strategies. I believe that this will rightly result in more microbiologists bridging between manufacturing and the laboratory where they can best leverage their thorough understanding of microbiology.
Outlook for 2023
In 2023 we will likely experience a great deal of discussion regarding the implementation of Annex 1 and contamination control. I have been privileged to be on the Parenteral Drug Association (PDA) task force for Annex 1 and Technical Report for Contamination Control Strategies, long awaited and due for publication (it may yet be out by the time you read this article). The USP may publish proposed draft chapters in microbial analytics in 2023 as there is much work to be done there. Lastly, I acknowledge that it is easy to sit back, contemplate and express these personal opinions, which may not be realised soon or ever. The difficulty today and always is that pharmaceutical microbiology is a demanding vocation frequently misunderstood and seldom recognised for its inherent challenges. I take my hat off to all pharmaceutical microbiologists who keep the pharmaceutical world turning; keep fighting the fight, finish the race and keep the faith.3
About the author
Edward C Tidswell, BSc, PhD is Executive Director within the Microbiological Quality and Sterility Assurance organisation of Merck with ownership over sterility assurance and microbiology issues across all sterile and non-sterile products and manufacturing. His prior appointments include senior global R&D, and quality leadership roles across diverse drug, device and biologics portfolios for the likes of Baxter Healthcare, Eli Lilly and Evans Vaccines. Edward actively publishes and is a leading authority on pharmaceutical microbiology, risk, aseptic and sterile manufacturing. Currently he is a member of the PDA Journal’s Editorial Board, and since June 2010 Dr Tidswell is honoured to serve on the USP Expert Committee for Microbiology.
References
- Stres B, Kronegger L. Shift in the paradigm towards next-generation microbiology. FEMS Microbiology Letters. 2019;366(15). Available from: https://doi.org/10.1093/femsle/fnz159.
- Jiang Y, Luo J, Huang D, et al. Machine learning advances in microbiology: A review of methods and applications. Frontiers in Microbiology. 2022;13. Available from: https://doi.org/10.3389/fmicb.2022.925454
- 1 Timothy 6:12. The Bible.
Issue
Related topics
Analytical techniques, Artificial Intelligence, Data Analysis, Drug Manufacturing, Drug Safety, Lab Automation, Manufacturing, Microbial Detection, Microbiology, Production, QA/QC, Recruitment, Regulation & Legislation










Thank you for this valuable information. This was lot of help for my assignment.