Data-enriched edible pharmaceuticals (DEEP): a new generation of solid drug products
Posted: 6 April 2022 | Ilari Ahola, Johan Bøtker, Jukka Rantanen, Natalja Genina, Sofia Kälvemark Sporrong | No comments yet
In this article, Natalja Genina, Ilari Ahola, Johan Bøtker and Jukka Rantanen at the University of Copenhagen and Sofia Kälvemark Sporrong from Uppsala University discuss the potential of data-enriched edible pharmaceuticals (DEEP) to overcome various drug administration and supply challenges.

Data-enriched edible pharmaceuticals (DEEP) are a novel concept for designing oral solid drug products (Figure 1). The unique feature of DEEP is that every single dosage form contains an individualised dose as well as embedded information that can be retrieved with a standard smartphone. This allows easy access to patient-specific information, recognition of the medicine and a better overview over the treatment.


Figure 1: The concept of data-enriched edible pharmaceuticals (DEEP): (A) fabrication of DEEP; (B) photographs of manufactured DEEP (size of each DEEP is 2cm by 2cm); (C) functionality of DEEP.
Current challenges in the pharmaceutical field
The current setup of conventional pharmaceutical manufacturing is based on mass production of a limited number of dosage strengths. This creates challenges, especially for the treatment of chronic critical diseases including cardiovascular diseases, type 2 diabetes as well as brain disorders. Many of the treatments for these diseases require an adjustment of dose(s) to the patient, based on the patient’s response to the medicine, co-administration of other medication, lifestyle changes, as well as going off medication. However, manipulation of the doses to maximise the therapeutic outcome is currently mainly done by either patients or care providers and can easily induce errors. Alternatively, it is done in compounding facilities, which is very laborious, time-consuming and expensive.
Another challenge that we are facing now is the absence of easily accessible patient-tailored information. For instance, it could regard the drug intake, especially when the administration regimen is complex and needs to be altered frequently, or when multiple drugs are to be taken. This can lead to non-optimal compliance and/or incorrect use of drugs and, consequently, to substandard therapeutic outcomes.
Finally, a rapid identification and/or authentication of the medicine is nearly impossible, if the secondary and primary package is lost or not available. It is also difficult to track and trace single dosage forms throughout the whole supply chain with the existing design of drug products.
Benefits of DEEP
These new drug products also give the opportunity for additional functionality such as digital traceability of a drug product along the supply chain, authenticity check and monitoring patients’ adherence to medicine”
The abovementioned challenges can be met by implementing DEEP, developed at the University of Copenhagen. In these products the data is presented, eg, in the pattern of a Quick Response (QR) code, which is printed with an ‘ink’ that contains a drug in patient-tailored doses and at the same time securely encapsulates information relevant to different stakeholders.1,2 The flexible adjustment of the dose is done by digital manipulation of the drug product design, eg, by selecting different patterns of the QR code. The unique physical appearance of QR code patterns in DEEP can help patients and other involved parties to identify medicines. Moreover, the personalised information, such as patient-specific administration instructions with schematic drawings, pictograms and videos, can be easily retrieved by scanning a QR code with a personal mobile device, such as a smartphone.
These new drug products also give the opportunity for additional functionality such as digital traceability of a drug product along the supply chain, authenticity check and monitoring patients’ adherence to medicine.3 There is an increasing focus on serialisation of medicinal products4,5 and introducing the concept of mass customization.6,7 In this context, DEEP can help to reduce, among others, drug shortages and stockpiling and give a better overview of produced, consumed and unused/expired drug products. They can also help in the sorting of medical waste, especially if the primary package is not available. This will support sustainability in the pharmaceutical area and create a basis for the circular pharmaceutical supply chain. DEEP technology also has the opportunity to interconnect the digital and pharmaceutical worlds by providing interactive personalised treatment.8
How DEEP are fabricated
Five main steps are involved in the fabrication of DEEP (Figure 1):
- Formulation of the printable ink that contains active substance(s), edible colouring agents and solvent/co-solvent systems
- Fabrication of edible ‘paper’ that is compatible with the ink
- Generation of the QR code that would encode the desired information via, for example, a password protected uniform resource locator (url)
- Scaling the QR code pattern to obtain the required (for the specific dose) number of pixels
- Printing of the ink onto the edible paper in the pattern of the QR code
The dose adjustment can be done digitally by altering (i) the pattern of the QR code with and without the embedded image, (ii) physical size of the QR code pattern and (iii) imprinting different number of subsequent layers (the higher the number of layers, the higher the dose is, Figure 2).
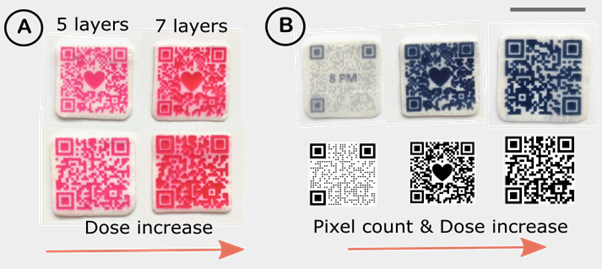

Figure 2: Digital adjustment of the dose by (a) changing the number of imprinted layers, and (b) by changing the coloured pixel count via varying the QR code pattern, embedding the image and changing the physical size of DEEP. The scale bar is 2cm.
Polymer coating on the QR pattern can be applied to avoid the direct contact between the patient and the active substance. This coating can also be used to modify the release of the drug from DEEP.3
Patient involvement in drug product design
The physical appearance of DEEP can be easily manipulated, as the QR code design is adjusted digitally. It means that patient preferences, such as size, pattern and colour, can be taken into account when designing the drug product. We have conducted interviews with patients regarding DEEP and, according to the received feedback, patients preferred DEEP in either a blue or red colour with a size that would not be bigger than the tongue. This is important, because patient involvement in the drug product design can improve compliance and adherence to medication.
Limitations of DEEP technology
At the moment, DEEP technology is more suitable for low-dose drugs that require frequent dose adjustment. This is related to product design challenges with the edible paper having a limited absorption capacity for the ink and, therefore, not being able to withhold a high volume of ink. On the other side, the concentration of the active substance in the ink is limited with its solubility in the selected solvents of the ink, which does not allow preparation of highly concentrated inks.
About the authors


Natalja Genina, Associate professor at University of Copenhagen (UCPH), Denmark. Her research interests include the fabrication of advanced drug delivery systems and their integration into digital health. Natalja has a remarkable publication record within her research field.


Ilari Ahola, PhD fellow at UCPH with the work focusing on DEEP.


Johan Bøtker, Associate professor at UCPH. Expert in image analysis.


Jukka Rantanen, Professor at UCPH. Expert in drug product design.


Sofia Kälvemark Sporrong, Professor at Uppsala University, Sweden. Expert in social pharmacy. Kälvemark Sporrong is a social scientist doing research within the area of pharmaceuticals. Her research interests include the patient perspective on drug use, pharmaceutical policy and professional ethics for health care staff.
Acknowledgement
The NordForsk for the Nordic University Hub project #85352 (Nordic POP, Patient Oriented Products) is acknowledged.
References
- Edinger M, Bar-Shalom D, Sandler N, et al. QR encoded smart oral dosage forms by inkjet printing. Int J Pharm. 2018 Jan 30;536(1):138–45.
- Öblom H, Cornett C, Bøtker J, et al. Data-enriched edible pharmaceuticals (DEEP) of medical cannabis by inkjet printing. Int J Pharm. 2020 Nov 15;589:119866.
- Chao M, Öblom H, Cornett C, et al. Data-Enriched Edible Pharmaceuticals (DEEP) with Bespoke Design, Dose and Drug Release. Pharm 2021, Vol 13, Page 1866 [Internet]. 2021 Nov 4 [cited 2021 Nov 9];13(11):1866. Available from: https://www.mdpi.com/1999-4923/13/11/1866/htm
- Snyder M. Experts Comment on FDA’s DSCSA Serialization Extension: Manufacturers Now Have Until 2018 – [Internet]. Pharmaceutical Processing World. 2017 [cited 2020 Oct 27]. Available from: https://www.pharmaceuticalprocessingworld.com/…
- Recommendations on a harmonised implementation of the EU Falsified Medicines Directive using GS1 standards [Internet]. The Global Language of Business. 2016 [cited 2021 Feb 1]. Available from: https://www.gs1.org/sites/default/files/docs/healthcare/…
- Siiskonen M, Mortensen NH, Malmqvist J, Folestad S. Adapting discrete goods supply chains to support mass customisation of pharmaceutical products. Concurr Eng [Internet]. 2021 Apr 1 [cited 2021 Apr 20];1063293X2110021. Available from: http://journals.sagepub.com/doi/10.1177/1063293X211002169
- Govender R, Abrahmsén-Alami S, Larsson A, Folestad S. Therapy for the individual: Towards patient integration into the manufacturing and provision of pharmaceuticals. Eur J Pharm Biopharm [Internet]. 2020 Apr 1 [cited 2021 Feb 9];149:58–76. Available from: https://pubmed.ncbi.nlm.nih.gov/31982577/
- Raijada D, Wac K, Greisen E, et al. Integration of personalized drug delivery systems into digital health. Adv Drug Deliv Rev. 2021 Sep 1;176:113857.
Related topics
Drug Delivery Systems, Drug Development, Drug Safety, Drug Supply Chain, Formulation, Informatics, Research & Development (R&D), Technology, Therapeutics