United efforts to accelerate drug development
Posted: 22 December 2021 | Prof Axel Glasmacher (University of Bonn), Prof Jaap Verweij MD PhD (Erasmus University Medical Centre), Prof John Smyth (University of Edinburgh) | No comments yet
From the Cancer Drug Development Forum (CDDF), John Smyth (Chairman), Axel Glasmacher (Treasurer) and Jaap Verweij (Managing Director) clarify the importance of a collaborative industry approach to cancer therapy development and highlight the most pressing challenges for discussion in their meetings and workshops.

The Cancer Drug Development Forum (CDDF) provides a unique opportunity for all stakeholders involved in developing anti-cancer medicines to analyse and debate the challenges posed by the rapid advances from scientific research versus the ever-increasing burden of cancer, and its associated costs. Workshops involving the pharma industry, researchers, regulators, health technology assessors and patients allow in-depth discussion to enhance understanding of obstacles with a view to progressing solutions for accelerated access to new effective medicines. Our workshops are supplemented by webinars delivered by experts – and for maximum participation we anticipate future meetings to involve hybrid interactions of in-person and on-line participation.
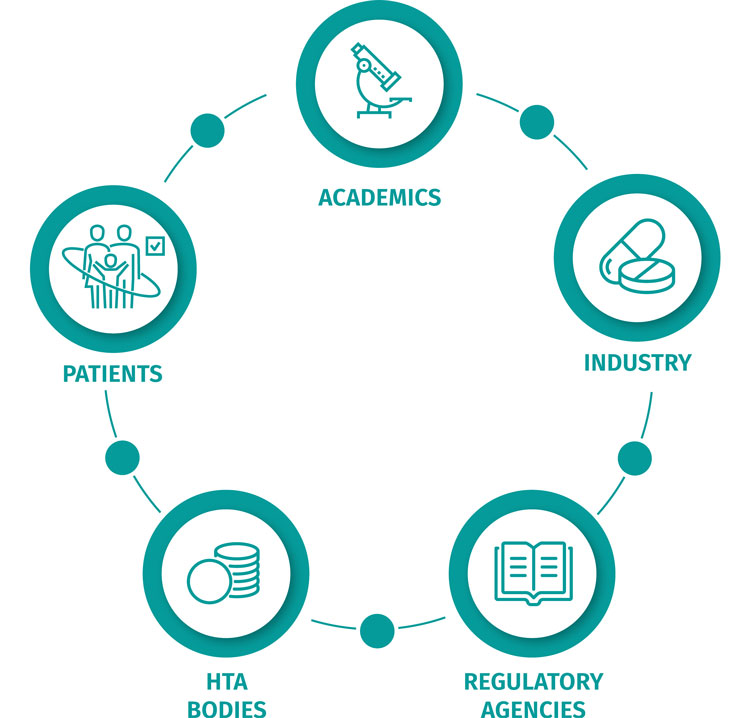

Multi-stakeholder, collaborative approach to accelerate the delivery of effective oncology treatments to patients.
The CDDF is a not-for-profit association based in Brussels whose aim is to accelerate the complex process of developing new medicines for patients with cancer. Our work is mainly aimed at European healthcare systems, but we have excellent relations with colleagues around the world since, despite solutions being focused on specific national conditions, the problems are universal.
The title ‘Forum’ was chosen deliberately to emphasise our non-political, non-financial and non‑critical approach to one of the great challenges of modern medicine. Scientific research has never been more productive in generating useful and translatable discoveries offering the potential to control more and more diseases. The problem is that as we control one set of conditions – cardiovascular disease or stroke, for example – the population lives longer, which results in increasing numbers of cancer patients. As we develop ever more successful drugs for these people, they may expect second-, third- or fourth‑line treatments, thereby greatly increasing the demand for all forms of healthcare, and obviously increasing the costs for the same number of patients. The costs of medicines are often singled out as the expensive part of overall treatment, but in reality, they are only part of a complex package of management issues.
Over the past two decades scientific progress has enabled us to identify with far greater precision the specific biological characteristics of different types of cancer and to design drugs that are far more selective for their given target. However, this apparent progress creates its own challenges for several reasons. While delivering greater efficiency for patients, it also fragments specific patient populations, thus reducing the scale of feasible clinical trials as fewer patients are available to identify success. This pressurises time to reach a decision about the value of a new intervention. Once a company is ready to submit a Marketing Authorisation Application (MAA), the regulators at the European Medicines Agency (EMA) or US Food and Drug Administration (FDA) must assess benefit versus safety in trials with many fewer patients than in the past, thus posing new statistical challenges.
The CDDF is a not-for-profit association based in Brussels whose aim is to accelerate the complex process of developing new medicines for patients with cancer.
The CDDF is particularly grateful for the enthusiastic participation that we receive from colleagues in both the EMA and FDA and recognise the helpful changes that have been introduced with various types of accelerated or conditional approvals. However, these changes in turn lead to further difficulties for the payers – the Health Technology Assessors (HTAs). While a license may allow sales worldwide, HTAs are country specific and understandably the public find it hard to accept that a new medicine is available in some locations and not others. Of all the stakeholders in drug development, the HTAs have the hardest decisions to make due to ‘opportunity costs’. If they allocate funds for one condition, with limited budgets they may not be able to address issues in other areas – and get criticised for their choices. The costs of new medicines are often criticised as well, defended by the companies as reflecting the high costs and risk of R&D, and the redundancy created by drugs falling off the pipeline during the pre-clinical and clinical journey. A highly contentious issue. Previously, anti-cancer drugs were developed on a general basis from identifying biological changes associated with malignancy. Broad spectrum clinical trials would seek to identify which, if any, specific cancer types responded, and then refine patient subsets with breast, colorectal, lung cancer, etc. This disease- or tissue-based approach created a lot of redundancy in the pipeline and took a long time to move from a laboratory-based discovery to a marketable drug – typically seven to 10 years. This absorbed up to 50 percent of the patent life of the drug, having a negative effect for the developers involved and contributing to the high cost of a new emergent medicine.


CDDF meetings to stimulate a productive dialogue and interaction between stakeholders in a neutral, non-competitive space.
The CDDF recognises each of these issues as genuine and challenging. To facilitate progress, we have established a series of meetings, workshops and, since the COVID-19 pandemic, on‑line webinars to address these problems, with the aim of increasing understanding between the various stakeholders and trying to identify areas where further progress can be made. We are particularly focused on working to accelerate timelines, using such initiatives as optimising clinical trial design (including patient selection), encouraging companies to meet with regulators early in their development plans, and helping regulators and HTAs to understand patients’ perspectives on what is needed from a particular new intervention. The latter point is especially important and, until relatively recently, not well appreciated. Survival at all costs is not necessarily the primary aim for many patients. The quality of that survival may override life expectancy and we now have instruments to record and analyse data generated by patients themselves.
Our workshops are held under ‘Chatham House Rules’ so that individuals can speak openly and not as representatives of any company or association. This often produces some very interesting results! Also, our workshops aim to provide a representation of views from all relevant stakeholders – namely, regulators, the pharmaceutical industry, academia, payers and especially important, patient advocates. With reference to some of the aforementioned topics, in April 2021 we held a workshop on “Endpoints in cancer drug development” which included a major discussion on Patient Reported Outcomes (PRO), which clearly pose challenges for regulators. Our programme for 2022 includes a workshop on patient engagement/access (19‑20 September), another on Tissue agnostic drug development (14-15 November) and a workshop on Measurable Residual Disease (25-26 April). The latter will review progress on technology’s contribution to drug development. Our increasing ability to detect cancer – either pre-clinically or identifying residual disease after treatment – is important in identifying who needs (further) treatment and who does not. Obviously, such knowledge has a profound impact not only on patients but on health services’ demands generally, which impacts costs.
Our future meetings will almost certainly be hybrids of in-person and on-line participation. The latter increases the possibility for colleagues to join us from different time zones and continents and we welcome all those interested to join us in this vital aspect of today’s cancer research – scientifically, really exciting for its potential, but very challenging for its optimum translation and delivery.
About the authors






Issue
Related topics
Anti-Cancer Therapeutics, Drug Development, Regulation & Legislation, Research & Development (R&D)
Related organisations
Cancer Drug Development Forum (CDDF), European Medicines Agency (EMA), US Food and Drug Administration (FDA)









