Attenuated diptheria toxin developed by researchers as novel form of drug delivery
Posted: 6 May 2020 | Victoria Rees (European Pharmaceutical Review) | No comments yet
A new potential platform for drug delivery has been created by researchers using attentuate diptheria toxin, which can enter cells and escape the endosome.
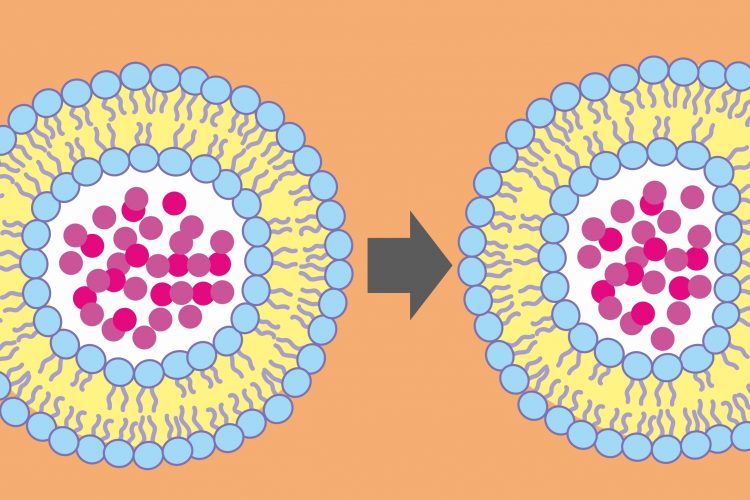

A group of researchers has developed a new potential way to deliver molecules that target specific genes within cells. Their platform, which uses a modified form of diptheria toxin, has been shown to downregulate critical genes in cancer cells and the researchers suggest it could be used for other genetic diseases as well.
The study was conducted at the University of Toronto Engineering and SickKids Hospital, both Canada.
“A major challenge in the field of drug delivery is that most therapeutic vehicles cannot escape the acid environment of the endosome once they get into the cell,” said Professor Molly Shoichet, the corresponding author of the research. “The diphtheria toxin platform as a delivery vehicle effectively solves that.”
According to the researchers, scientists investigating how to place molecules inside cells have a number of existing tools to choose from, but most suffer from the same drawback – while the molecule gets inside the cell, it remains trapped in an endosome. If the goal is to deliver therapeutics that will interact with the cell’s DNA, breaking out of the endosome is critical.
As a natural defence mechanism, bacteria such as Corynebacterium diptheriae produce a protein-based toxin that enters surrounding cells, eventually killing them. Critically, this toxin is known to be capable of escaping from endosomes, which led to the idea of re-engineering it as a delivery platform.

Modified diptheria toxin used to reduce the activity of genes connected to the propagation of brain cancer [credit: Shoichet Lab].
The researchers developed a non-toxic version of the diphtheria toxin known as attenuated diptheria toxin. This new molecule has the capacity to enter the cell and efficiently escape the endosome, making it a potential delivery vehicle without any of the toxic effects of the diphtheria toxin.
To prove that the concept would work, the researchers used the system to deliver molecules they believed would be effective against glioblastoma in cell cultures.
“Glioblastoma is a highly invasive disease and patients have a very short life expectancy after initial diagnosis,” said Shoichet, “We want to change this and have thus pursued the delivery of gene therapeutics to treat glioblastoma.”
The group first targeted glioblastoma neural stem cells, which are thought to be resistant to chemotherapeutics. Specifically, the researchers focused on delivering silencing RNA (siRNA) against two genes: integrin beta 1 (ITGB1), which is associated with the highly invasive nature of glioblastoma (and other cancers) and eukaryotic translation initiation factor 3 subunit b (eIF-3b), which is an essential survival gene. By eliminating this invasive trait, the researchers could potentially limit progression in diseases like cancer.
“ITGB1 is involved in cancer cell migration, which contributes to glioblastoma’s invasion into healthy brain tissues,” said Laura Smith, a senior PhD student on the publication. “We used an innovative three-dimensional culture system to significantly reduce cell invasion after treatment with our siRNA-attenuated diptheria toxin system, which suggests that it may be effective in slowing disease progression.”
To demonstrate the breadth of this platform, the researchers also delivered a different nucleic sequence that knocks down eIF-3b, which participates in the ‘survival pathway’ of cancer cells.
“We treated the cells with the attenuated diphtheria toxin-siRNA against eIF-3b and observed down-regulation at genetic and phenotypic levels,” said Dr Amy Arnold, from the Shoichet lab and first author on this paper.
The group is planning on using this delivery vehicle to treat other diseases in the future.
“We recognise the strength of this platform strategy and are actively testing it for the delivery of RNA and other cargoes,” concluded Shoichet.
The study was published in Science Advances.
Related topics
Anti-Cancer Therapeutics, Drug Delivery Systems, Preclinical Research, Research & Development (R&D)