The COVID-19 pandemic and the Indian pharmaceutical industry
Posted: 22 April 2020 | Dr Abhishek Dadhich (Assistant Professor at IIHMR University - Jaipur) | 11 comments
Dr Abhishek Dadhich rounds up how the Indian pharmaceutical market has been impacted by the coronavirus pandemic and the steps the country’s government could take to overcome these setbacks.


The Indian pharmaceutical industry is the world’s third largest drug producer by volume and the country’s market manufactures 60 percent of vaccines globally. This constitutes 40 to 70 percent of supply to satisfy the World Health Organization’s (WHO) demand for Diphtheria, Tetanus and Pertussis (DPT) and Bacillus Calmette Guerin (BCG) vaccines and 90 percent of the global demand for the measles vaccine.
India supplies affordable and low-cost generic drugs to millions of people around the globe and operates more than 250 US Food and Drug Administration (FDA) and UK Medicine and Healthcare products Regulatory Agency (MHRA) approved plants. Furthermore, its active pharmaceutical ingredients (APIs) market is forecasted to attain a revenue of $6 billion by the end of 2020.
According to a report on the Indian pharmaceutical industry, the source of APIs is a crucial part of the pharma industry’s strategic plan to combat the COVID-19 pandemic. The majority of APIs for generic drug manufacturing across the globe are sourced from India, which also supplies approximately 30 percent of the generic APIs used in the US. However, Indian manufacturers rely heavily on APIs from China for the production of their medicine formulations, procuring around 70 percent from China, the top global producer and exporter of APIs by volume.
The impact of the SARS-CoV-2 coronavirus outbreak, COVID-19, has exposed the dependency of the Indian pharma sector on China for its API procurement. Supply chain disruptions and product exportation restrictions from India resulted from manpower shortages in China’s manufacturing plants. This was caused by the quarantine policies adapted and adopted by different provincial governments in China in response to the virus. Supplies were further impacted by the disruption of logistic and transportation systems, restricting access and movement of products to and from ports.
Prior to 1991, the Indian pharmaceutical industry only imported 0.3 percent of its APIs from China; however, the globalisation of Indian pharmaceutical companies and the rise in large-scale formulation manufacturing prompted the increase in API procurement from China. The primary driver being the low cost of production.
The current dependency of Indian pharmaceutical companies on Chinese APIs is a serious concern for national health security, prompting the Indian government to set up a taskforce to review the internal API sector. Several key representatives from the pharmaceutical industry and NITI Aayog (an Indian government policy think tank) suggested that fostering the approvals of pharmaceutical infrastructure developments, clearance from the environment ministry and providing tax exemptions and subsidies for the development and promotion of the pharmaceutical industry hubs could all benefit the market.
Strategies for promoting Indian API production
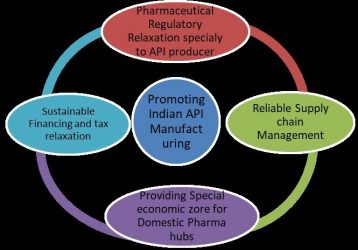

Diagram of the steps the Indian government could take to promote domestic API manufacturing.
In the present situation, the Indian government should take important steps to remove the technical and financial barriers that will spur the pharmaceutical industry to ramp up API production, reducing the dependency of the pharmaceutical industry as a whole on the heavily impacted Chinese market. The Indian government recently undertook applaudable steps by proposing an incentive package of 13.76 billion Indian Rupees (approximately $181 million) for the promotion of domestic manufacturing of critical key starting materials, drug intermediates, APIs and medical devices.
The Indian pharmaceutical industry maintains great advantages, including the availability of a large labour pool and advanced technologies that enable high regulatory standards of markets like the US and European countries to be met.
About the author:
Dr Abhishek Dadhich is a Revolutionist Academic Researcher and Trainer in the area of Pharmaceutical Management. Dr Abhishek is presently working as Assistant Professor at the Indian Institute of Health Management Research (IIHMR) University, Jaipur. He has more than 11 years of experience in academic research and training and has contributed more than 15 research, review papers and articles to international and national journals of repute, pharma magazines, websites and a blog.
References:
- Chatterjee, P. Indian pharma threatened by COVID-19 shutdowns in China [Internet]. The Lancet. 29 February 2020. [Cited: 19 March 2020]. Available at: https://www.thelancet.com/journals/lancet…
- European Pharmaceutical Review. India should reduce dependence on China for APIs, says industry insider [Internet]. European Pharmaceutical Review. 13 March 2020. [Cited: 22 March 2020] Available at: https://www.europeanpharmaceuticalreview.com/news/115163…
- James, T.India’s Growing Dependence on Imports in the area of Bulk Drugs [Internet]. RIS. February 2015. [Cited: 21 March 2020]. Available at: https://www.ris.org.in/sites/default/files/…
- ET Burea. ICRA downgrades Indian pharma on China lockdown [Internet]. Economic Times. 21 February 2020. [Cited: 16 March 2020]. Available at: https://economictimes.indiatimes.com/markets/…
- Arnum, P. Indian Government Looks To Increase Domestic API Mfg [Internet]. DCAT. 25 March 2020 [Cited: 25 March 2020] Available at: https://dcatvci.org/6411-indian-government…
- PIB Delhi. Cabinet approves Promotion of domestic manufacturing of critical Key Starting Materials/Drug Intermediates and Active Pharmaceutical Ingredients in the country [Internet]. PIB Delhi. 21 March 2020. [Cited: 25 March 2020]. Available at: https://pib.gov.in/PressReleasePage…
Related topics
Active Pharmaceutical Ingredient (API), Drug Manufacturing, Drug Markets, Drug Supply Chain, Funding, Generics, Regulation & Legislation, Sustainability, Vaccines, Viruses
Related organisations
Indian Institute of Health Management Research (IIHMR) University, NITI Aayog, UK Medicine and Healthcare products Regulatory Agency (MHRA), US Food and Drug Administration (FDA), World Health Organization (WHO)










Thank you for sharing such an informative article. Loved reading this.
Amazing write-up
very nice information.
That was an insightful article. I like the concept of promoting our Indian API rather depending on other countries. Keep posting
Information is pretty good and impressed me a lot. This article is quite in-depth and gives a good overview of the topic.
Rigorous research on the virus to understand it better – how it spreads, its journey within our bodies- for different age groups, with or without co-morbidities and symptoms; the impact of different drug combinations, how different temperatures impact it, its interaction with various surfaces; the actual cause of death in Covid positive patients, is necessary for a solution to combat the disease. This research which is on at various levels is a must in exploring other routes to prevention and cure. We need to also leverage alternate systems of medicines to find solutions across, increasing immunity or reducing the severity of symptoms.
I believe, as in the past, human ingenuity will find mechanisms to keep ourselves safe and learn to live with it until such time that a vaccine is ready.
very good information sir
Very Good information Sir. India Pharma industry will surely have bright future.
Very nice post with good quality information. I liked the strategy for promoting Indian API production. Keep sharing informative posts like this.
Thanks for your appreciation
A nice post.
Brief but explanatory.
Would like to read few more papers by the author.