Test method validation for cleaning validation samples
Posted: 19 March 2008 | Richard Forsyth (Merck) | 2 comments
Testing cleaning validation samples requires a validated method. The extent of validation is dependent upon the type of method employed, the capabilities of the method, the scientific and regulatory needs of the resulting data and the anticipated outcome of the testing. A number of test method options are reviewed for their analytical capabilities, along with their method validation parameters.


Cleaning validation is a critical function in pharmaceutical manufacturing. Regulatory agencies have placed great emphasis on demonstrating that a cleaning process prevents cross-contamination1,2. Manufacturing equipment cleanliness does not merely impact the subsequent formulation, but every formulation processed in the equipment and the overall manufacturing program in a facility. A cleaning process is validated and monitored through testing of the equipment. Testing ranges from visual inspection to swab sampling or rinse sampling.
For any test method to be suitable for its intended purpose, it must be appropriate for measuring analytes at and below the acceptable residue limit (ARL). An ARL can be based on available toxicological data such as an allowable daily intake (ADI), an adulteration limit such as 10 ppm and visual cleanliness. The ARL has a direct bearing on the validation parameters of the test method.
For any test method to be suitable for its intended purpose, it must be appropriate for measuring analytes at and below the acceptable residue limit
The two main types of sampling are direct surface sampling with swabs, which is most desirable and finally, rinse sampling1. Cleaning validation test results can be expressed as a limit test or cover a range of analyte concentration. The testing of the samples can consist of a specific method such as high performance liquid chromatography (HPLC), gas chromatography (GC), mass spectrometry (MS) or a non-specific method such as total organic carbon (TOC), pH and conductivity. The type of sample, expected results and assay methodology used for testing also affect the determination and demonstration of the validation parameters.
Validation parameters
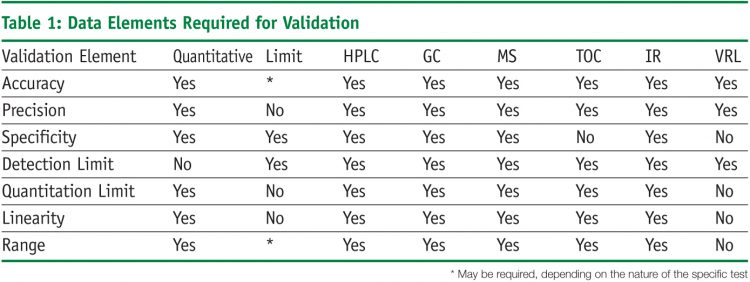
The analytical performance characteristics or validation parameters as defined by the USP3 include: accuracy, precision, specificity, detection limit, quantitation limit, linearity, range and robustness. Validation elements differ for a method that covers a range or a limit (Table 1). Validation of a limit test requires fewer resources, but the data results from testing provide less information. Addressing these performance characteristics provides assurance that a method meets proper standards of accuracy and reliability.

Validation study
Prepare a solution of the residue of interest at a concentration from which spots of appropriate size can be prepared. For example, pipet 200 µl of a solution with a concentration of five times the ARL. Inoculate in triplicate residue spots of known concentrations at 3-5 levels around the ARL on coupons of the material of construction of the manufacturing equipment. Wetted swabs recover the residue from the surface and solvent extracts the residue from the swab.
The use of HPLC for the testing of cleaning validation samples is well established and can address all validation parameters
The recovery data demonstrates accuracy, precision, linearity and range. LOD and LOQ can be determined experimentally or estimated from the lowest sample recovered. Standard and sample solution stability should be determined experimentally. Specificity and robustness are dependent on the method employed.
Methodology
There are a wide variety of methods available to test cleaning validation samples. The method of choice is often the one with which we have familiarity. Methods such as pH and conductivity can provide cleaning data and require only instrument calibration. Many methods require validation
HPLC
The use of HPLC for the testing of cleaning validation samples is well established4-7 and can address all validation parameters. HPLC is a chromatographic method that involves a sample in a liquid stream that passes through a packed column and separates from the other components of the sample. An HPLC method can separate the residue of interest from the components of the formulation as well as the detergent. A well designed HPLC recovery study can demonstrate accuracy, precision, linearity, range, LOD and LOQ in a single run.
Typically, HPLC methods for assay are designed to quantitate levels down to 0.1% of the active pharmaceutical ingredient (API), making sensitivity well below most calculated ARLs. However, the sensitivity of HPLC is dependent on the chemical structure of the residue of interest and the detector, which can quantitate the residue. A residue with no UV chromophore requires either a specialised detector (fluorescence, electrochemical) or derivatisation to achieve the desired sensitivity.
HPLC assay methods can be lengthy, 30-40 minutes per injection, which could be an issue for quick turnaround of samples.
Gas chromatography
Gas chromatography has also been used to test cleaning validation samples8. This methodology is analogous to HPLC with comparable selectivity and sensitivity. The sample carrier is in a stream of gas which passes through a column for separation and a detector. Detectors are not limited to compounds with UV chromophores. The validation parameters and their demonstration are similar to HPLC. Gas chromatography is limited to residues that are volatile at the temperatures generated in the instrument.
Mass spectrometry
A mass spectrometer (MS) is an instrument that measures the atomic mass of a sample. There are several types of MS instruments but the atmospheric ionisation models are widely used for cleaning validation.
An MS detector can be coupled with an HPLC9,or cleaning samples can be inserted directly10. It separates the residue of interest through mass selectivity from the components of the formulation and the detergent. This method of analysis is sensitive down to the ppm or ppb level and a recovery study can demonstrate accuracy, precision, linearity, range, LOD and LOQ in a single run.
An MS detector can be coupled with an HPLC, or cleaning samples can be inserted directly
An accurate linear range can be limited, unless an internal standard is employed. An internal standard is a compound of similar chemical structure to the residue of interest, which will react in the same manner in the MS. Without the internal standard, accuracy can be restricted to concentrations at or near the ARL.
HPLC, GC and MS all require method development effort prior to validation. Method development can be fairly straightforward or extremely difficult, depending on the residue of interest and the sample matrix. Therefore, method development can range from a few days to several months.
In many cases method development will have occurred during another phase of the program development such as release assay, residual solvents or structural elucidation. Often the method selected for cleaning validation is the result of these previous efforts.
Total organic carbon
Total organic carbon analysis is also well established for testing cleaning validation samples
Total organic carbon (TOC) analysis is also well established for testing cleaning validation samples11-16. TOC analysis involves the oxidation of carbon and the detection of the resulting carbon dioxide produced from the oxidation reaction. Sensitivity is down to the ppm or ppb level. Method development for TOC is straightforward in that the analysis method is consistent and the sample preparation changes slightly. A well designed TOC recovery study can also demonstrate accuracy, precision, linearity, range, LOD and LOQ in a single run.
TOC is a non-specific method of analysis. All organic carbon is detected. Therefore, any residue detected must be considered the residue of interest. Residues for TOC analysis must dissolve in water. This can limit the effective linear range of the residue assay.
Infrared
Infrared (IR) testing of cleaning validation samples monitors absorbance of the residue in the infrared region of the spectrum. Studies have demonstrated sensitivity down to the ppm or ppb level17,18 as well as accuracy, precision, linearity, range, LOD and LOQ. This methodology is designed to take readings directly from the equipment surface, resulting in a very quick turnaround of clean equipment.
Selectivity can be an issue if different residues have similar IR absorbance and analogous to HPLC, not all residues demonstrate the necessary absorbance in the IR region.
Visible residue
Visual inspection using a visible residue limit (VRL) can monitor equipment cleaning efficiency down to the ppm or ppb level19,20. The VRL is the level below which the residue of interest is not visible to the equipment inspector. Results from validation activity can be used to establish the difference between the ARL and the VRL. Since this is a limit test, the detection limit is the primary validation parameter. Precision is also determined by using multiple observers in order to minimise subjectivity of the observers. This testing is also designed to make observations directly from the clean equipment for quick turnaround.
Results from validation activity can be used to establish the difference between the ARL and the VRL
A VRL inspection is non-specific. Any visible residue is considered unacceptable. Subjectivity and training of the inspectors is the primary concern with this process. Several of its applications and associated risks have been demonstrated21.
Conclusions
The choice of testing methodology and validation parameters for cleaning validation depends on the specific combination of facility, equipment and formulations. There are a variety of viable methods to test cleaning validation samples. Validation of the testing methodology for cleaning validation samples can be accomplished efficiently. Proper validation of the cleaning validation sample test method(s) helps assure that the cleaning program will be acceptable to regulatory scrutiny.
USP validation parameters
- Accuracy – closeness of test results to the true value across the range.
- Precision – degree of agreement among individual test results applied repeatedly to multiple samples of a homogeneous sample.
- Specificity/Selectivity – ability to assess the analyte in the presence of components expected to be present.
- Detection Limit (LOD) – the lowest amount of analyte that can be detected under the stated experimental conditions.
- Quantitation Limit (LOQ) – the lowest amount of analyte that can be determined with acceptable precision and accuracy under the stated experimental conditions.
- Linearity – the ability to elicit test results that are proportional to the concentration of the analyte within a given range.
- Range – the interval between the upper and lower levels of analyte that have been demonstrated to be determined with a suitable level of precision, accuracy, and linearity.
- Robustness – the measure of its capacity to remain unaffected by small but deliberate variations in procedural parameters.
References
- FDA, “Guide to Inspection of Validation of Cleaning Processes,” (Division of Field Investigations, Office of Regional Operations, Office of Regulatory Affairs, July 1993).
- Annex 15 to the EU Guide to GMP, Brussels, July 2001.
- United States Pharmacopeia Vol 30, National Formulary Vol. 25, 680-683 (2007)
- A. H. Schmidt, “Validated HPLC Method for the Determination of Residues of Acetaminophen, Caffeine, and Codeine Phosphate on Swabs Collected from Pharmaceutical Manufacturing Equipment in Support of Cleaning Validation,” J.Liq. Chrom.& Rel. Technol. 29, 1663-1673 (2006).
- M. A. Boca, Z. Apostolides and E. Pretorius, “A Validated HPLC Method for Determining Residues of a Dual Active Ingredient Anti-Malarial Drug on Manufacturing Equipment Surfaces,” J. Pharm. & Biomed. Anal. 37 461-468 (2005).
- R. J. Forsyth and D. Haynes, “Cleaning Validation in a Pharmaceutical Research Facility,” Pharm. Technol.22 (9), 104- 112 (1998).
- P. Yang, K. Burson, et. al., “Method Development of Swab Sampling for Cleaning Validation of a Residual Active Pharmaceutical Ingredient,” Pharm. Technol.29 (1), 84- 94 (2005).
- T. Mirza, R. George, et. al., “Capillary Gas Chromatography Assay of Residual Methenamine Hippurate in Equipment Cleaning Validation Swabs,” J. Pharm. & Biomed. Anal. 16 939-950 (1998).
- K. J. Kolodsick, H. Phillips, et. al., “Enhancing Drug development by Applying LC-MS-MS for Cleaning Validation in Manufacturing Equipment,” Pharm.Technol. 30 (2) 56-72 (2006).
- K. Payne, W. Fawber, et. al., “IMS for Cleaning Validation,” Role of Spectros. PAT January 2005.
- A. H. Mollah, “Risk-Based Cleaning Validation in Biopharmaceutical API Manufacturing,” BioPharm Inter .30 (11), 54- 68 (2005).
- K. M Jenkins, A. J. Vanderwielen, et. al., “Application of Total Organic Carbon Analysis to Cleaning Validation,” PDA J.Pharm. Sci.& Technol. 50 (1) 6-15 (1996).
- A. Walsh, “Using TOC Analysis for Cleaning Validation,” presented at The Validation Council’s conference of cleaning validation, Princeton, N.J. Oct. 27, 1999.
- B. Wallace, R. Stevens and M. Purcell, “Implementing Total Organic Carbon Analysis for Cleaning validation,” Pharm. Technol.Aseptic Processing 40-43 (2004)
- C. Glover, “Validation of the Total Organic Carbon (TOC) Swab Sampling and Test Method,” PDA J.Pharm. Sci.& Technol. 60 (5) 284-290 (2006).
- P. Bristol, “Cleaning Validation the Rise of TOC,” Manufac. Chem. 37-38 April 2004
- N. Teelucksingh and K. B. Reddy, “Quantification of Active Pharmaceutical Ingredients on Metal Surfaces Using a Mid-IR Grazing-Angle fiber Optics Probe – An In-Situ Cleaning Verification Process, Spectros. 20 (10) 16-23 (2005).
- N. Mehta, J. Goenaga-Polo, et. el., “Development of an In Situ Spectroscopic Method for Cleaning Validation Using Mid-IR Fiber-Optics,” BioPharm Inter. (8) 54-68 (2002).
- R. J. Forsyth and V. Van Nostrand, “Visible Residue Limit for Cleaning Validation and its Potential Application in a Pharmaceutical Research Facility,” Pharm. Technol.28 (10) 58-72 (2004).
- R. J. Forsyth and V. Van Nostrand, “Application of Visible Residue Limit for Cleaning Validation in a Pharmaceutical Manufacturing Facility,” Pharm. Technol. 29 (10) 152-161 (2005).
- R. J. Forsyth, J. Hartman and V. Van Nostrand, “Risk-Management Assessment of Visible-Residue Limits in Cleaning Validation,” Pharm. Technol. 30 (9) 101-114 (2006).
About the author
Richard Forsyth is an Associate Director with GMP quality in Merck & Co., Inc. He is responsible for internal and external facility audits as well as document audits for regulatory submissions. Richard has worked in Quality for two years, prior to this he worked for 23 years as an Analytical Chemist in Pharmaceutical R&D. He has been involved with Cleaning Validation for over 14 years. Mr. Forsyth has a broad range of analytical experience including methods development and validation, as well as formulation development and project management. Academic training includes an MS in Chemistry and an MBA in Management, both from St. Joseph’s University in Philadelphia, Pennsylvania, USA.









Nice article, as i am chemist, i was looking for information about cleaning validation this article help me to get more clarity on cleaning validation.
Can you suggest how to proceed for accuracy test where drug is highly volatile and evaporate at room temperature for example nitroglycerin