Once-a-month oral contraceptive pill one step closer
Posted: 5 December 2019 | Victoria Rees (European Pharmaceutical Review) | No comments yet
A new slow-release formulation and delivery system for the oral contraceptive pill has shown success in pre-clinical trials and could be taken once a month.


Researchers have developed a new formulation and system of drug delivery for the oral contraceptive pill which could be taken once a month. Having shown success in pre-clinical trials, the team are now undertaking safety evaluations.
The team, from Brigham and Women’s Hospital and the Massachusetts Institute of Technology (MIT), US built upon previous research to create slow-release pills that can reside in the stomach for days or weeks.
…work is now underway to bring the extended-release pill closer to human trials”
“Our capsule represents a major advancement toward providing women with a once-a-month contraceptive. For many, this may be hard to believe. But our pre-clinical data is encouraging us along that road,” said co-corresponding author Dr Giovanni Traverso, a gastroenterologist and physician-researcher at the Brigham and MIT. “We began our work on extended drug release by working with treatments for malaria, tuberculosis and HIV. But early on, we were having conversations about the potential impact that extended drug release could have for family planning.”
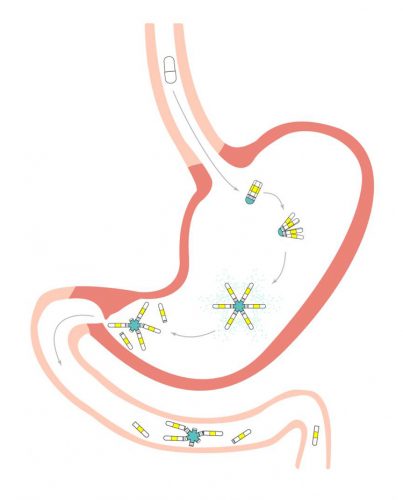

Investigators have designed a drug-delivery vehicle that consists of six arms joined by an elastic-coated core. The arms were loaded with the oral contraceptive drug levonorgestrel and folded up into a capsule that can be swallowed. Once in the stomach, the arms unfold and have a span that is larger than the opening of the human pylorus, helping the system stay in the stomach where it can release the drug over time (credit: Lyndra Therapeutics).
The team designed a drug-delivery vehicle that consists of six arms joined by an elastic-coated core. The arms were loaded with the oral contraceptive drug levonorgestrel and folded up into a capsule that can be swallowed. Once in the stomach, the arms unfold and have a span that is larger than the opening of the human pylorus, helping the system stay in the stomach where it can release the drug over time.
The researchers tested the concentration of oral contraceptive over time in a pig model and measured the presence of the drug in the bloodstream for animals who had been given the extended-release form versus an immediate release tablet. For the tablet, dosage tapered off after six hours. For the extended-release form, the team observed concentrations of the drug for up to 29 days.
According to the researchers, work is now underway to bring the extended-release pill closer to human trials. The next steps will include scaling up manufacturing processes and safety evaluations.
The findings were published in Science Translational Medicine.
Related topics
Drug Delivery Systems, Drug Development, Formulation, Preclinical Research, QA/QC, Research & Development (R&D)
Related organisations
Brigham and Women’s Hospital, Massachusetts Institute of Technology (MIT)





![Close up view of the Merck logo on the top corner of a glass building [Credit: Michael Vi / Shutterstock.com].](https://www.europeanpharmaceuticalreview.com/wp-content/uploads/Merck-aquisition-400x187.jpg)



