Mylan announces voluntary recall of Alprazolam tablets
Posted: 29 October 2019 | Victoria Rees (European Pharmaceutical Review) | No comments yet
Due to possible contamination from a foreign substance, Mylan Pharmaceuticals is voluntarily recalling one batch of its Alprazolam tablets.

The US Food and Drug Administration (FDA) has announced that Mylan Pharmaceuticals is conducting a voluntary recall of Alprazolam tablets. The recall is due to potential contamination from a foreign substance.
The 0.5mg tablet lot being recalled were distributed from between July 2019 and August 2019. Each bottle contains 500 tablets.
The recall is nationwide in the US at the consumer level. The medication is for the treatment of anxiety disorder and panic disorder.
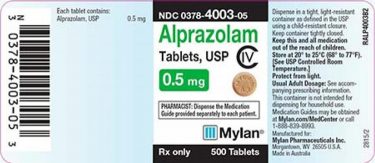

The packaging from the affected batch (credit: US FDA).
Although the clinical impact from the foreign material is expected to be rare, there is still a risk of infection to patients. Currently, no adverse events related to this batch have been reported.
The pharmaceutical company has announced it is arranging for the return of all the products. The recalled drugs should be quarantined and any distribution discontinued.
Related topics
Related organisations
Mylan Pharmaceuticals, US Food and Drug Administration (FDA)









