ITC: affinity is not everything
Posted: 7 February 2009 | Dr Ernesto Freire, Johns Hopkins University | No comments yet
During the optimisation of drug candidates, improvements in affinity and selectivity play a critical role. This task is usually accomplished by establishing accurate correlations between the affinity/selectivity of different chemical scaffolds and through chemical modifications to a selected scaffold.
During the optimisation of drug candidates, improvements in affinity and selectivity play a critical role. This task is usually accomplished by establishing accurate correlations between the affinity/selectivity of different chemical scaffolds and through chemical modifications to a selected scaffold.
During the optimisation of drug candidates, improvements in affinity and selectivity play a critical role. This task is usually accomplished by establishing accurate correlations between the affinity/selectivity of different chemical scaffolds and through chemical modifications to a selected scaffold.
Isothermal titration calorimetry (ITC) is universally acknowledged as the technique that provides the most accurate measurements of binding affinity. It is the gold standard against which other techniques are compared. ITC, however, is not only able to measure binding affinity but also the magnitude of the different thermodynamic forces that determine the binding energy. Since different chemical functionalities contribute differently to the binding forces, the knowledge acquired by ITC also provides precise guidelines for optimisation of drug candidates.
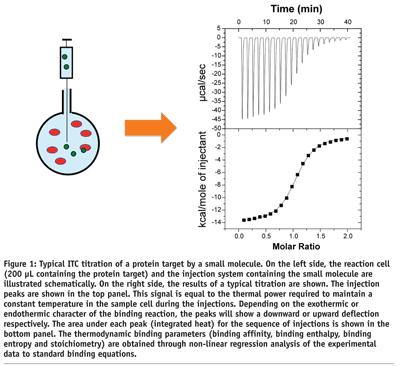
Isothermal titration calorimetry determines the binding of a molecule to another by directly measuring the heat that is either absorbed (endothermic) or released (exothermic) when the binding reaction takes place. In modern ITC instruments (see Figure 1 on page 46) the binding reaction takes place in a reaction cell (200 µL in the most modern instrument) where one of the reactants (usually the macromolecule) is placed. T he binding reaction is triggered by the injection of the second reactant into the reaction cell (~1.4 µL per injection)1. Proper mixing is ensured by continuously stirring the solution. A complete titration is generated by the stepwise injection of the second reactant until saturation of the macromolecule is achieved. In the design of an ITC experiment, the concentration of reactants is calculated such that complete saturation occurs in fifteen injections or less. The heat associated with each injection is proportional to the amount of complex that is formed. As the macromolecule becomes saturated, the heat effects diminish in magnitude as fewer and fewer ligand molecules bind to the target. After saturation, additional injections produce small identical peaks, due to non-specific phenomena such as dilution and mixing effects, whose heat effect must be subtracted from all the injection peaks before performing data analysis. Analysis of the ligand concentration dependence of the heat effect for each injection leads to the determination of the binding affinity2-4. Because the experimental observable is the heat of reaction, the analysis also yields the binding enthalpy and the binding entropy for the reaction. These two quantities provide unique information about the forces that dominate the binding reaction.
ITC is the only technique that is capable of measuring simultaneously the binding affinity (Ka) and the enthalpy (DH) and entropy (DS) changes associated with the binding reaction. A typical ITC experiment consumes approximately 1 nmol of macromolecule (i.e. 20 ug for a protein of 20 kDa) and can be performed in less than one hour. ITC provides this unique capability in solution, in a label-free format, and without the need to immobilise either the target macromolecule or ligand2. Having access to the enthalpy and entropy changes, in addition to the binding constant, greatly accelerates the optimisation process.
The binding affinity
The binding affinity of a drug candidate for its target is the result of a combination of different forces. Attractive forces between the drug molecule and target, of which van der Waals and hydrogen bonds are the most prominent; and repulsive forces that tend to drive the drug out of the aqueous solvent into macromolecule cavities devoid of water, of which the hydrophobic effect is the most important. In both cases, shape complementarity between the drug molecule and the binding cavity in the target plays a critical role since the strength of van der Waals interactions depends on how closely the ligand molecule fits within the binding pocket. Shape complementarities are also critical when binding is driven by hydrophobicity since a close fit maximizes desolvation and improves selectivity. The strength of hydrogen bonds, on the other hand, depends on the distance and angle between hydrogen bond acceptors and donors in the drug and target molecules. For binding to occur these favorable binding forces need to overcome the unfavorable forces associated with the restriction of translational/rotational and conformational degrees of freedom that occur when the drug binds to its target. The combined contributions of all these favorable and unfavorable forces give rise to the Gibbs energy of binding (DG) which in turn defines the binding affinity Ka = e -DGRT-. Maximal binding affinity occurs when DG is very large and negative.
The Gibbs energy of binding is made up of two contributions, the enthalpy (DH) and entropy (DS) changes (DG = DH – TDS). Consequently extremely high affinity is only achieved when both, enthalpy (DH) and entropy (DS) contribute optimally to binding5-8. Accordingly, assessing the enthalpy/entropy balance of a drug candidate is critical for the design of optimisation strategies. For example, if a compound were already entropically optimised, the affinity optimisation goal would require the improvement of the binding enthalpy. The converse is true for a compound that is enthalpically optimised. It is clear that it would be advantageous to know the binding enthalpy and entropy changes of a drug candidate; so accurate optimisation strategies can be devised.
During the optimisation of drug candidates, there are four binding forces that can be engineered in order to improve binding affinity:
- van der Waals interactions
- Hydrogen bonds
- Hydrophobic
- Conformational constraints aimed at minimizing conformational entropy losses
Maximal affinity is achieved when all these forces are optimised and contribute favorably to binding. It is apparent that optimisation would be faster and more efficient if the forces that require extra consideration could be identified. This is where ITC plays a unique role because different forces contribute differently to the enthalpy and entropy changes, the two quantities that can be measured by this technique; van der Waals interactions and hydrogen bonds contribute favorably to the binding enthalpy, while hydrophobicity contributes primarily to favorable binding entropy. The introduction of conformational constraints, on the other hand, minimizes unfavorable conformational entropy effects. Entropic effects linked to hydrophobicity or to conformational changes can be distinguished by their effect on the heat capacity change, i.e. the temperature dependence of the enthalpy change9.

Thermodynamic signature and optimisation
The enthalpic and entropic contributions to the binding energy can be visualised in the thermodynamic signature of a compound (see Figure 2). This plot provides an immediate snapshot of the enthalpy/entropy balance of a compound and the goals that need to be accomplished for optimisation. In the example in Figure 2 on page 47, one is dealing with a compound that binds with an affinity close to 50 nM (DG = -10 kcal/mol). The binding is dominated by a large favorable entropy (-TDS = -15 kcal/mol) and an unfavorable binding enthalpy (DH = 5 kcal/mol). A large favorable binding entropy is characteristic of compounds that bind with a large hydrophobic component, whereas an unfavorable enthalpy usually indicates the presence of polar groups that become buried from the solvent without establishing a strong interaction (e.g. hydrogen bond) with the target. The desolvation penalty is stronger than any favorable interaction. The thermodynamic signature in Figure 2 on page 47 immediately suggests optimisation approaches. For example, modest aims like lowering the unfavorable enthalpy from 5 to 2 kcal/mol will improve the binding affinity from 50 nM to 0.3 nM, i.e. to the high picomolar level. ITC can help to establish clear optimisation goals.
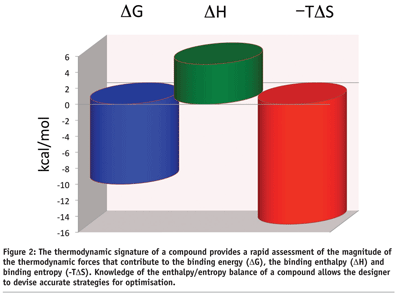

Thermodynamic signatures can be used to select the most promising compounds among hits identified by high throughput screening. The characterisation obtained by the thermodynamic signature goes beyond a simple affinity ranking since it also provides important insights into the binding mechanism of the various compounds. This information is critical since many times a compound can be preferred due to a more robust binding mechanism than simply to a better binding affinity during screening.
During optimisation it is common to observe that the enthalpy change becomes unfavorable, especially after the introduction of polar groups. This phenomenon is an indication that a polar group becomes buried and desolvated but does not establish a strong hydrogen bond, the enthalpic contribution being dominated by the unfavorable desolvation enthalpy10. Also, the introduction of hydrogen bonds is often compensated by opposing entropic changes resulting in no affinity gains11. ITC is the only technique that will identify the reasons for the lack of improvement and suggest corrective actions.
Extremely high binding affinity is achieved when both the enthalpy and entropy changes contribute favorably to binding. The evolution of the HIV-1 protease inhibitors provides a clear picture. First generation protease inhibitors like indinavir are characterised by unfavorable enthalpy changes while newer inhibitors have strongly favorable enthalpies5,12,13. A similar situation has been observed for the cholesterol lowering drugs, the statins14.
Summary
ITC provides the most accurate measurements of binding affinity and has become the gold standard to gauge the accuracy of other techniques. In addition, ITC is also the only technique capable of measuring the thermodynamic components of the binding energy, the enthalpy and entropy changes. ITC does that in one experiment with the reagents free in solution and without the requirement of labels or reporter groups. As the use of ITC becomes widespread in the pharmaceutical industry, significant breakthroughs in lead optimisation can be expected in the near future.
References
- Brandts, J.M., Brown, R.K., O’Brien, R. & Peters, W.P. ITC-Derived Binding Constants: Using Microgram Quantities of Protein. in Label-Free Biosensors: Techniques and Applications (ed. Cooper, M.) (Cambridge University Press 2007).
- Wiseman, T., Williston, S., Brandts, J.F. & Lin, L.N. Rapid measurement of binding constants and heats of binding using a new titration calorimeter. Anal. Biochem. 179, 131-135 (1989).
- Straume, M. & Freire, E. Two-Dimensional Differential Scanning Calorimetry: Simultaneous Resolution of Intrinsic Protein Structural Energetics and Ligan Binding Interactions by Global Linkage Analysis. Anal. Biochem. 203, 259-268 (1992).
- Velazquez Campoy, A. & Freire, E. ITC in the post-genomic era…? Priceless. Biophys Chem 115, 115-24 (2005).
- Ohtaka, H. & Freire, E. Adaptive Inhibitors of the HIV-1 protease. Progr. Biophys. Mol. Biol. 88, 193-208 (2005).
- Carbonell, T. & Freire, E. Binding thermodynamics of statins to HMG-CoA reductase. Biochemistry 44, 11741-8 (2005).
- Ruben, A.J., Kiso, Y. & Freire, E. Overcoming roadblocks in lead optimization: a thermodynamic perspective. Chem Biol Drug Des 67, 2-4 (2006).
- Sarver, R.W. et al. Binding thermodynamics of substituted diaminopyrimidine renin inhibitors. Anal Biochem 360, 30-40 (2007).
- Luque, I. & Freire, E. A system for the structure-based prediction of binding affinities and molecular design of peptide ligands. Methods. Enzymol 295, 100-127 (1998).
- Cabani, S., Gianni, P., Mollica, V. & Lepori, L. Group contributions to the thermodynamic properties of non-ionic organic solutes in dilute aqueous solution. J. Solution Chem. 10, 563-595 (1981).
- Lafont, V. et al. Compensating enthalpic and entropic changes hinder binding affinity optimization. Chem Biol Drug Des 69, 413-22 (2007).
- Velazquez-Campoy, A., Kiso, Y. & Freire, E. The Binding Energetics of First and Second Generation HIV-1 Protease Inhibitors: Implications for Drug Design. Arch. Biochim. Biophys. 390, 169-175 (2001).
- Ohtaka, H. et al. Thermodynamic Rules for the Design of High Affinity HIV-1 Protease Inhibitors with Adaptability to Mutations and High Selectivity Towards Unwanted Targets. Int. J. Biochem. Cell Biol 36, 1787-1799 (2004).
- Carbonell, T. & Freire, E. Binding thermodynamics of statins to HMG-CoA reductase. Biochemistry 44, 11741-11748 (2005).




