FDA approves Abbott’s molecular Zika virus test
Posted: 3 February 2017 | | No comments yet
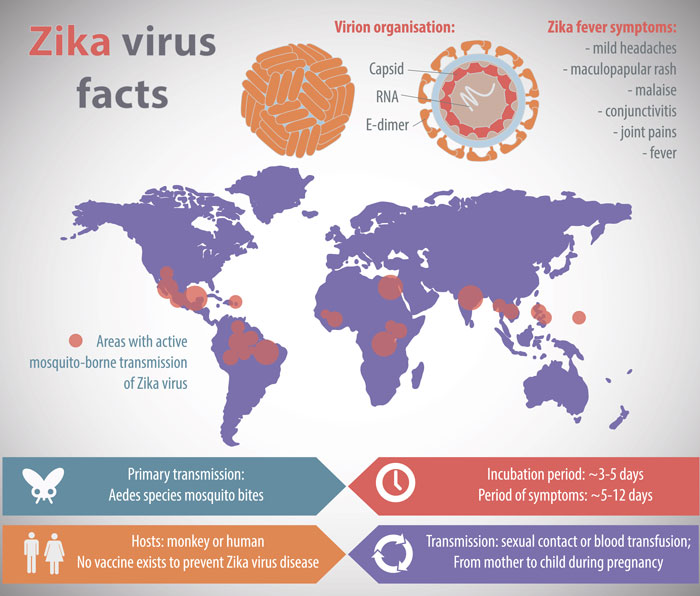
The US Food and Drug Administration (FDA) has authorised Abbott’s molecular test to detect Zika virus in whole blood (when collected alongside a patient-matched serum or plasma sample) for emergency use. This is the first molecular test made by a commercial manufacturer authorised to detect Zika in whole blood samples; this is significant since the […]

The US Food and Drug Administration (FDA) has authorised Abbott’s molecular test to detect Zika virus in whole blood (when collected alongside a patient-matched serum or plasma sample) for emergency use.

This is the first molecular test made by a commercial manufacturer authorised to detect Zika in whole blood samples; this is significant since the Zika virus can be detected in whole blood for a longer period of time (up to two months) and at higher levels, versus testing with serum and urine sample types.
“Diagnosing a Zika infection can be challenging, especially since people might not have any symptoms or only have mild symptoms that last a few days,” said John Hackett, Abbott.
“Abbott’s molecular test may provide the ability to identify the active virus over a longer time period with whole blood and could provide a more accurate diagnosis. Our test can also distinguish Zika from other viruses such as dengue or chikungunya, which helps doctors make informed diagnoses to help people get back to better health.”
Providing results within five to seven hours, the test is highly sensitive to detect if someone is infected with Zika. It is also automated, allowing people who work in the lab to be more efficient and spend less time preparing and handling samples, reducing the chances of error and increasing speed to diagnosis.
Abbott’s Zika research and development
Abbott has several additional R&D projects underway (currently in development and not yet FDA approved, cleared or authorised) to help address testing needs related to the current Zika outbreak.
- Today’s existing tools to detect Zika and other tropical diseases are laboratory-based and require reliable power sources, but often, testing is needed in remote areas where there are no labs. To address this issue, the US Defense Advanced Research Projects Agency (DARPA) awarded a contract to Abbott to develop a testing panel for Zika and multiple tropical fever pathogens for use on a mobile platform to meet the needs of testing in rural and remote areas.
- Another issue to address is the development of serology tests that do not cross-react with other tropical disease antibodies. Through a grant from the US Agency for International Development (USAID), Abbott is exploring the development of a serology test to solve this challenge.
According to the World Health Organization, Zika remains a significant enduring public health challenge. It is primarily spread to people through bites from infected mosquitoes but can also be passed from pregnant women to their foetuses or through sexual transmission.