Digital simulations could advance nanoparticle drug delivery
Posted: 2 July 2024 | Catherine Eckford (European Pharmaceutical Review) | No comments yet
Findings from the mathematical model could support development of personalised treatments, the research suggests.
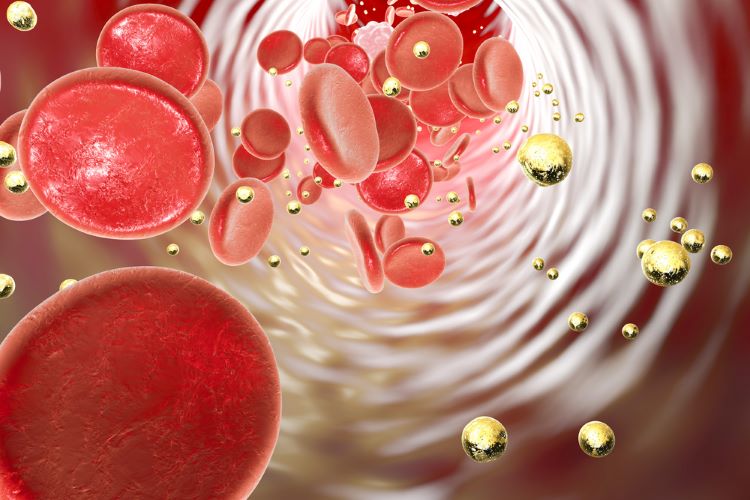

Researchers have developed a technique to optimise drug delivery using nanoparticles. They applied computer simulations based on blood flow dynamics, to investigate the effects of blood flow on the adhesion and retention of nanoparticle drug carriers.
Studies investigating drug delivery via the bloodstream to diseased tissues “have been effective” when “using mouse models and in vitro tissue models”, yet these have used nanoparticles designed “mostly by trial and error”, the researchers emphasised.
[this study presents] first kind of demonstration where there is a more systematic, robust design of nanoparticles, under the guidance of physics”
Notably, the study described here is “the first kind of demonstration where there is a more systematic, robust design of nanoparticles, under the guidance of physics,” explained Hyunjoon Kong, a Robert W Schafer Professor in the department of chemical & biomolecular engineering, Carle Illinois College of Medicine.
To attract nanocarriers from the bulk blood flow to the endothelial cell surface, the researchers used protein-protein attraction instead of a magnetic attraction. Kong’s researchers coated the nanocarrier with the same protein expelled by the diseased tissue at the target site.
They then injected nanoparticles “at a rate that replicated the arterial system and then flushed during a wash cycle to determine the concentration of remaining particles”.
The model simulations, done via supercomputers, can be conducted in 24 to 48 hours. This shortens the timeline to optimise a treatment protocol significantly. For example, typically it can take months to years, according to Goraya et al.
Effects on nanoparticle size
When simulating the effect of nanoparticle size, larger particles were found to perform better at adhesion and retention at the endothelial layer.
One of the key findings was the study showed “a significant loss of particles due to external flow for small diameter nanoparticles”, Kong stated.
Specifically, 200nm particles had detachment issues and “would be washed away with external flow”. Conversely, increasing the diameter to 1000nm made the nanoparticles “too large for transport”. In the experiment, the researchers concluded that 700nm was optimal for attaching particles at the vascular wall.
Future applications
“The model is very general and can be applied to any kind of disease, different shapes of nanoparticles and different drugs,” Arif Masud, Professor of Mechanics and Computations, Department of Civil and Environmental Engineering, University of Illinois Urbana-Champaign added.
Positively, based on the study the team noted that they can “create a digital twin of a living human to optimise the drug for that patient,” Masud shared. Overall, this means the model can support development of personalised treatments.
This research, published in Proceedings of the National Academy of Sciences, was funded by the National Institutes of Health (NIH).
Related topics
Drug Delivery Systems, Drug Development, Industry Insight, Personalised medicine, Proteins, Research & Development (R&D), Technology, Therapeutics